Monday Poster Session
Category: IBD
P3309 - Long-Term Safety of Ustekinumab vs Risankizumab in Biologic-Naive Inflammatory Bowel Disease Patients: Insights From a Retrospective Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Omar Arman, MD, MPH
University at Buffalo
Depew, NY
Presenting Author(s)
Omar Arman, MD, MPH1, Louis Hobeika, MD2, Omar Daas, MD3, Lan Nguyen, DO1, Abdulrahman Arman, MD3, Khaled Rafeh, MD4, Mazen Zamzam, BS5, Jad Bou-Abdallah, MD1
1University at Buffalo, Buffalo, NY; 2University of Jordan, Amman, 'Amman, Jordan; 3University of Jordan School of Medicine, Amman, 'Amman, Jordan; 4School of Medicine, The University of Jordan, Shmeisani, 'Amman, Jordan; 5Oakland University William Beaumont School of Medicine, Royal Oak, MI
Introduction: Biologic agents have revolutionized the management of inflammatory bowel disease (IBD) over the past two decades. While these therapies effectively induce and maintain remission, understanding their safety profiles is essential for long-term management. This study compares the long-term safety of ustekinumab and risankizumab in biologic-naive IBD patients, focusing on key adverse events.
Methods: Using the TriNetX database, we identified biologic-naive IBD patients who initiated ustekinumab or risankizumab between January 2020 and December 2023 and had no prior cancer history. Propensity score matching balanced baseline age, gender, race, and disease severity, forming two cohorts: ustekinumab (n = 329) and risankizumab (n = 329). Safety outcomes, including cancers, infections, thromboembolism, and hospitalizations, were evaluated at 3 and 5 years. Kaplan-Meier analysis assessed the probabilities of remaining free from adverse events.
Results: At 3 years, infection-free probabilities were lower for risankizumab (42.7%) compared to ustekinumab (72.2%, p = 0.345), despite similar infection event rates (19.5% vs. 18.2%). Cancer-free probabilities remained comparable between the two cohorts (95.3% for ustekinumab vs. 92.6% for risankizumab, p = 0.400). Similarly, thromboembolism-free and hospitalization-free probabilities showed no significant differences. These trends persisted at 5 years (Figures 1 and 2), with no statistically significant differences observed in overall safety outcomes. However, the notable and consistent gap in infection-free probabilities warrants further investigation to clarify its clinical relevance.
Discussion: The 3- and 5-year follow-up data indicate that ustekinumab and risankizumab have comparable safety profiles, with no significant differences in rates of cancer, thromboembolism, or hospitalizations. However, the lower infection-free probability for risankizumab highlights a potential trend that may become clinically relevant with larger sample sizes or longer follow-up. These findings support the safe, long-term use of both biologics but underscore the need for individualized safety monitoring, particularly for infection risk. Future studies should explore whether these differences reflect inherent drug properties or incidental findings.
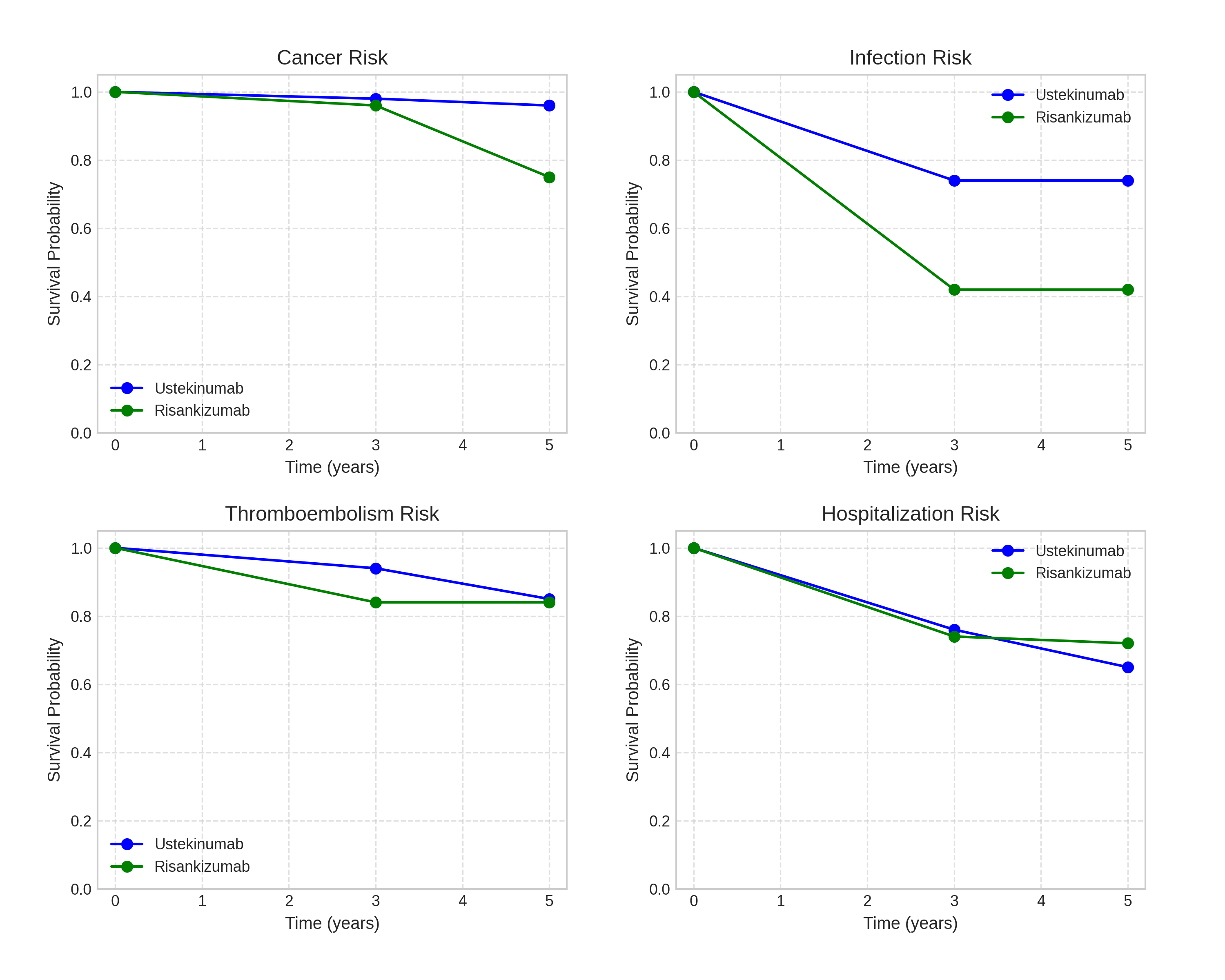
Figure: Figure 1: Kaplan-Meier survival curves showing probabilities of remaining free from adverse events (cancer, infections, thromboembolism, hospitalizations) for ustekinumab (Stelara) and risankizumab (Skyrizi) cohorts over 5 years.
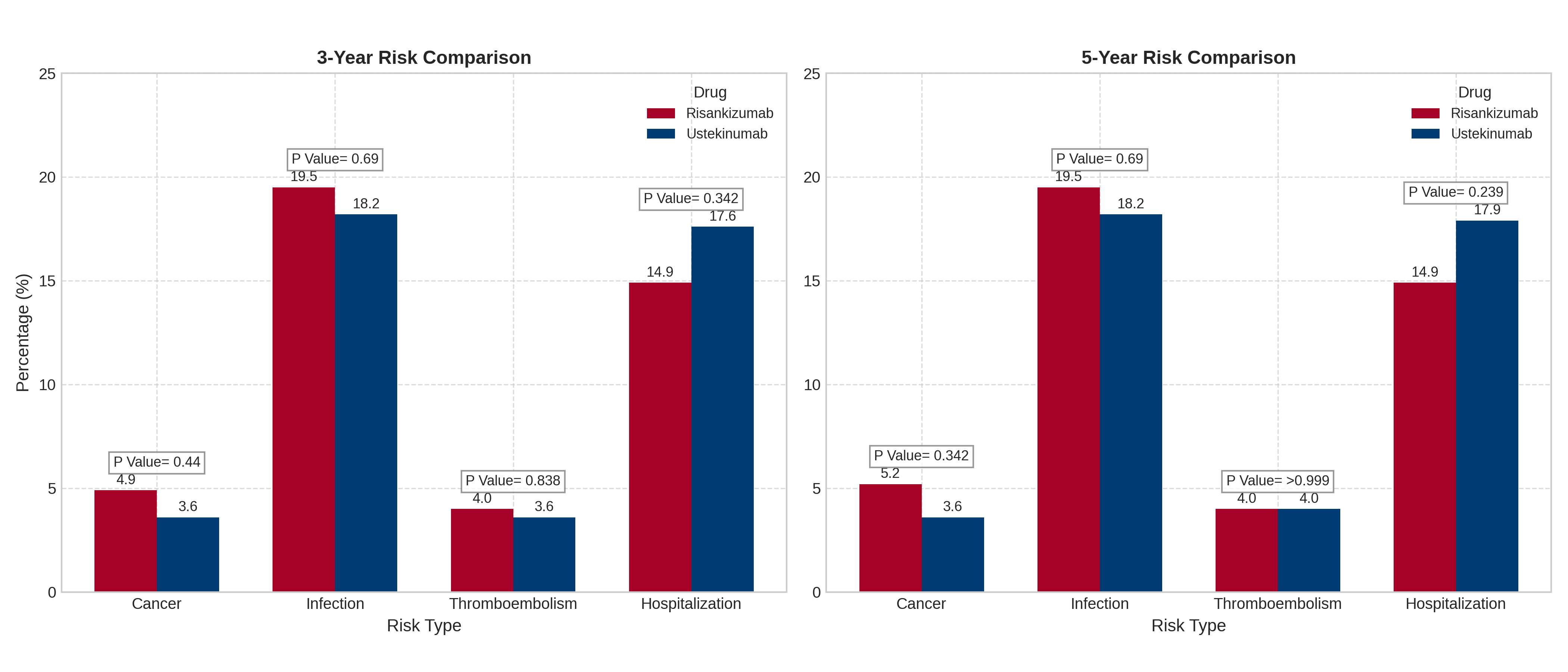
Figure: Figure 2: Comparison of 3-year and 5-year risk percentages for Cancer, Infection, Thromboembolism, and Hospitalization between patients treated with risankizumab (Skyrizi) and ustekinumab (Stelara). P-values indicate the statistical significance of the difference between the two treatments for each risk type at each time point.
Disclosures:
Omar Arman indicated no relevant financial relationships.
Louis Hobeika indicated no relevant financial relationships.
Omar Daas indicated no relevant financial relationships.
Lan Nguyen indicated no relevant financial relationships.
Abdulrahman Arman indicated no relevant financial relationships.
Khaled Rafeh indicated no relevant financial relationships.
Mazen Zamzam indicated no relevant financial relationships.
Jad Bou-Abdallah indicated no relevant financial relationships.
Omar Arman, MD, MPH1, Louis Hobeika, MD2, Omar Daas, MD3, Lan Nguyen, DO1, Abdulrahman Arman, MD3, Khaled Rafeh, MD4, Mazen Zamzam, BS5, Jad Bou-Abdallah, MD1. P3309 - Long-Term Safety of Ustekinumab vs Risankizumab in Biologic-Naive Inflammatory Bowel Disease Patients: Insights From a Retrospective Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University at Buffalo, Buffalo, NY; 2University of Jordan, Amman, 'Amman, Jordan; 3University of Jordan School of Medicine, Amman, 'Amman, Jordan; 4School of Medicine, The University of Jordan, Shmeisani, 'Amman, Jordan; 5Oakland University William Beaumont School of Medicine, Royal Oak, MI
Introduction: Biologic agents have revolutionized the management of inflammatory bowel disease (IBD) over the past two decades. While these therapies effectively induce and maintain remission, understanding their safety profiles is essential for long-term management. This study compares the long-term safety of ustekinumab and risankizumab in biologic-naive IBD patients, focusing on key adverse events.
Methods: Using the TriNetX database, we identified biologic-naive IBD patients who initiated ustekinumab or risankizumab between January 2020 and December 2023 and had no prior cancer history. Propensity score matching balanced baseline age, gender, race, and disease severity, forming two cohorts: ustekinumab (n = 329) and risankizumab (n = 329). Safety outcomes, including cancers, infections, thromboembolism, and hospitalizations, were evaluated at 3 and 5 years. Kaplan-Meier analysis assessed the probabilities of remaining free from adverse events.
Results: At 3 years, infection-free probabilities were lower for risankizumab (42.7%) compared to ustekinumab (72.2%, p = 0.345), despite similar infection event rates (19.5% vs. 18.2%). Cancer-free probabilities remained comparable between the two cohorts (95.3% for ustekinumab vs. 92.6% for risankizumab, p = 0.400). Similarly, thromboembolism-free and hospitalization-free probabilities showed no significant differences. These trends persisted at 5 years (Figures 1 and 2), with no statistically significant differences observed in overall safety outcomes. However, the notable and consistent gap in infection-free probabilities warrants further investigation to clarify its clinical relevance.
Discussion: The 3- and 5-year follow-up data indicate that ustekinumab and risankizumab have comparable safety profiles, with no significant differences in rates of cancer, thromboembolism, or hospitalizations. However, the lower infection-free probability for risankizumab highlights a potential trend that may become clinically relevant with larger sample sizes or longer follow-up. These findings support the safe, long-term use of both biologics but underscore the need for individualized safety monitoring, particularly for infection risk. Future studies should explore whether these differences reflect inherent drug properties or incidental findings.

Figure: Figure 1: Kaplan-Meier survival curves showing probabilities of remaining free from adverse events (cancer, infections, thromboembolism, hospitalizations) for ustekinumab (Stelara) and risankizumab (Skyrizi) cohorts over 5 years.

Figure: Figure 2: Comparison of 3-year and 5-year risk percentages for Cancer, Infection, Thromboembolism, and Hospitalization between patients treated with risankizumab (Skyrizi) and ustekinumab (Stelara). P-values indicate the statistical significance of the difference between the two treatments for each risk type at each time point.
Disclosures:
Omar Arman indicated no relevant financial relationships.
Louis Hobeika indicated no relevant financial relationships.
Omar Daas indicated no relevant financial relationships.
Lan Nguyen indicated no relevant financial relationships.
Abdulrahman Arman indicated no relevant financial relationships.
Khaled Rafeh indicated no relevant financial relationships.
Mazen Zamzam indicated no relevant financial relationships.
Jad Bou-Abdallah indicated no relevant financial relationships.
Omar Arman, MD, MPH1, Louis Hobeika, MD2, Omar Daas, MD3, Lan Nguyen, DO1, Abdulrahman Arman, MD3, Khaled Rafeh, MD4, Mazen Zamzam, BS5, Jad Bou-Abdallah, MD1. P3309 - Long-Term Safety of Ustekinumab vs Risankizumab in Biologic-Naive Inflammatory Bowel Disease Patients: Insights From a Retrospective Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
