Monday Poster Session
Category: IBD
P3308 - Comparing Infliximab and Adalimumab for Biomarker-Based Remission in Crohn’s Disease: Insights From Real-World Data
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Omar Arman, MD, MPH
University at Buffalo
Depew, NY
Presenting Author(s)
Omar Arman, MD, MPH1, Laith M.. Haj-Ahmad, MD2, Khaled Rafeh, MD3, Husam Abu Suilik, MD4, Amer Arman, MD5, Hebah Jaber, MD6, Noor Arman, MD7, Mazen Zamzam, BS8, Jad Bou-Abdallah, MD1
1University at Buffalo, Buffalo, NY; 2University of Jordan, Amman, 'Amman, Jordan; 3School of Medicine, The University of Jordan, Shmeisani, 'Amman, Jordan; 4The Hashemite University, Zarqa, Az Zarqa', Jordan; 5MedStar Health, Washington, WA; 6Mutah University, Mutah, Al Karak, Jordan; 7University of Jordan School of Medicine, Amman, 'Amman, Jordan; 8Oakland University William Beaumont School of Medicine, Royal Oak, MI
Introduction: Infliximab and Adalimumab are cornerstone therapies for Crohn’s disease (CD), yet direct comparisons of their efficacy in achieving biomarker-based remission are limited. With the use of the TriNetX database, one of the largest and most diverse real-world cohorts to date, this study evaluates remission induction outcomes for these biologic agents, offering insights into their roles in CD management.
Methods: We conducted a retrospective cohort study using TriNetX data from 93 healthcare organizations. A total of 16,291 adult patients (≥18 years) with CD treated with Infliximab or Adalimumab between 2018 and 2023 were initially identified. Propensity score matching was performed to balance age, sex, race/ethnicity, disease severity, and comorbidities, resulting in 6,516 matched CD patients per treatment group (total n = 13,032). The primary outcome was biomarker-based remission, defined as serum C-reactive protein (CRP) ≤5 mg/L or fecal calprotectin ≤150 µg/g, and assessed at 6 months, 1 year, 3 years, and 5 years. Induction of remission rates and Kaplan-Meier survival analyses were reported for the CD cohort.
Results: Infliximab demonstrated consistently higher remission rates than Adalimumab across all time points (p < 0.001). At 6 months, remission was achieved more frequently with Infliximab in CD (38.5% vs 28.3%). This pattern was held at 1 year (49.8% vs 42.6%), 3 years (66.1% vs 62.7%), and 5 years (71.2% vs 68.5%). Hazard ratios (HR) were directionally standardized to reflect Infliximab over Adalimumab (Infliximab/Adalimumab). Therefore, HR > 1 indicates faster remission with Infliximab. Infliximab was associated with a significantly shorter time to remission across all time points in CD (all HRs > 1, p < 0.001). Median time to remission was also consistently shorter with Infliximab compared to Adalimumab across the CD group. This supports the hazard ratio findings and further shows a faster induction of remission with Infliximab. Table 1 shows a detailed breakdown of these statistics.
Discussion: Infliximab achieves more frequent and faster remission rates for CD at all time points. This may help guide biologic therapy selection and early treatment decisions in CD.
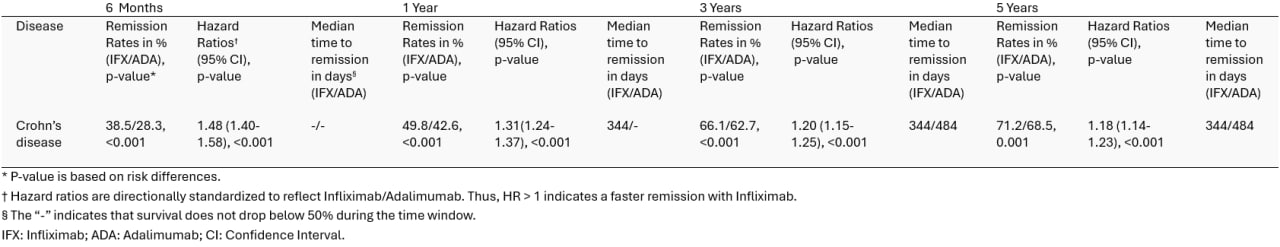
Figure: Table 1. Remission rates, hazard ratios, and median time to remission for Infliximab versus Adalimumab in CD patients from 6 months to 5 years.
Disclosures:
Omar Arman indicated no relevant financial relationships.
Laith Haj-Ahmad indicated no relevant financial relationships.
Khaled Rafeh indicated no relevant financial relationships.
Husam Abu Suilik indicated no relevant financial relationships.
Amer Arman indicated no relevant financial relationships.
Hebah Jaber indicated no relevant financial relationships.
Noor Arman indicated no relevant financial relationships.
Mazen Zamzam indicated no relevant financial relationships.
Jad Bou-Abdallah indicated no relevant financial relationships.
Omar Arman, MD, MPH1, Laith M.. Haj-Ahmad, MD2, Khaled Rafeh, MD3, Husam Abu Suilik, MD4, Amer Arman, MD5, Hebah Jaber, MD6, Noor Arman, MD7, Mazen Zamzam, BS8, Jad Bou-Abdallah, MD1. P3308 - Comparing Infliximab and Adalimumab for Biomarker-Based Remission in Crohn’s Disease: Insights From Real-World Data, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University at Buffalo, Buffalo, NY; 2University of Jordan, Amman, 'Amman, Jordan; 3School of Medicine, The University of Jordan, Shmeisani, 'Amman, Jordan; 4The Hashemite University, Zarqa, Az Zarqa', Jordan; 5MedStar Health, Washington, WA; 6Mutah University, Mutah, Al Karak, Jordan; 7University of Jordan School of Medicine, Amman, 'Amman, Jordan; 8Oakland University William Beaumont School of Medicine, Royal Oak, MI
Introduction: Infliximab and Adalimumab are cornerstone therapies for Crohn’s disease (CD), yet direct comparisons of their efficacy in achieving biomarker-based remission are limited. With the use of the TriNetX database, one of the largest and most diverse real-world cohorts to date, this study evaluates remission induction outcomes for these biologic agents, offering insights into their roles in CD management.
Methods: We conducted a retrospective cohort study using TriNetX data from 93 healthcare organizations. A total of 16,291 adult patients (≥18 years) with CD treated with Infliximab or Adalimumab between 2018 and 2023 were initially identified. Propensity score matching was performed to balance age, sex, race/ethnicity, disease severity, and comorbidities, resulting in 6,516 matched CD patients per treatment group (total n = 13,032). The primary outcome was biomarker-based remission, defined as serum C-reactive protein (CRP) ≤5 mg/L or fecal calprotectin ≤150 µg/g, and assessed at 6 months, 1 year, 3 years, and 5 years. Induction of remission rates and Kaplan-Meier survival analyses were reported for the CD cohort.
Results: Infliximab demonstrated consistently higher remission rates than Adalimumab across all time points (p < 0.001). At 6 months, remission was achieved more frequently with Infliximab in CD (38.5% vs 28.3%). This pattern was held at 1 year (49.8% vs 42.6%), 3 years (66.1% vs 62.7%), and 5 years (71.2% vs 68.5%). Hazard ratios (HR) were directionally standardized to reflect Infliximab over Adalimumab (Infliximab/Adalimumab). Therefore, HR > 1 indicates faster remission with Infliximab. Infliximab was associated with a significantly shorter time to remission across all time points in CD (all HRs > 1, p < 0.001). Median time to remission was also consistently shorter with Infliximab compared to Adalimumab across the CD group. This supports the hazard ratio findings and further shows a faster induction of remission with Infliximab. Table 1 shows a detailed breakdown of these statistics.
Discussion: Infliximab achieves more frequent and faster remission rates for CD at all time points. This may help guide biologic therapy selection and early treatment decisions in CD.

Figure: Table 1. Remission rates, hazard ratios, and median time to remission for Infliximab versus Adalimumab in CD patients from 6 months to 5 years.
Disclosures:
Omar Arman indicated no relevant financial relationships.
Laith Haj-Ahmad indicated no relevant financial relationships.
Khaled Rafeh indicated no relevant financial relationships.
Husam Abu Suilik indicated no relevant financial relationships.
Amer Arman indicated no relevant financial relationships.
Hebah Jaber indicated no relevant financial relationships.
Noor Arman indicated no relevant financial relationships.
Mazen Zamzam indicated no relevant financial relationships.
Jad Bou-Abdallah indicated no relevant financial relationships.
Omar Arman, MD, MPH1, Laith M.. Haj-Ahmad, MD2, Khaled Rafeh, MD3, Husam Abu Suilik, MD4, Amer Arman, MD5, Hebah Jaber, MD6, Noor Arman, MD7, Mazen Zamzam, BS8, Jad Bou-Abdallah, MD1. P3308 - Comparing Infliximab and Adalimumab for Biomarker-Based Remission in Crohn’s Disease: Insights From Real-World Data, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
