Monday Poster Session
Category: IBD
P3273 - Effects of Risankizumab on the Extraintestinal Manifestations of Crohn's Disease
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Hannah O'Neill, MD (she/her/hers)
Houston Methodist-Weill Cornell Graduate School of Medical Sciences
Houston, TX
Presenting Author(s)
Hannah O'Neill, MD1, Oscar Noble, MD2, Poojasree Pasupuleti, MS2, Bincy Abraham, MD, MS, FACG3, Christopher Fan, MD4
1Houston Methodist-Weill Cornell Graduate School of Medical Sciences, Houston, TX; 2Houston Methodist Hospital, Division of Gastroenterology and Hepatology, Lynda K. and David M. Underwood Center for Digestive Health / Houston Methodist Research Institute, Department of Medicine, Houston, TX; 3Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX; 4Houston Methodist Hospital, Division of Gastroenterology and Hepatology, Lynda K. and David M. Underwood Center for Digestive Health / Houston Methodist Research Institute, Department of Medicine / Weill Cornell Medical College, Cornell University, Houston, TX
Introduction: Crohn’s disease (CD), characterized by chronic transmural inflammation of the GI tract, can lead to strictures & fistulas, obstruction, & cancer. Additionally, a wide range of debilitating extra-intestinal manifestations (EIMs) can occur. EIM response to CD treatment is variable & does not always coincide with IBD response. Biologic options for CD treatment now include risankizumab (RZB), an interleukin-23 inhibitor (IL-23i). This analysis aimed to assess RZB effects on EIMs & determine any baseline characteristics that predict response.
Methods: This retrospective, single center study included CD patients receiving RZB from 2022 to 2024. Baseline characteristics were examined to determine predictors of EIM response. Variables included demographics, markers of disease location / severity (Montreal Classification, fecal calprotectin, inflammatory markers), & prior medical therapies. Clinical parameters included Harvey-Bradshaw Index & Simple Endoscopic Score for CD. Exclusion criteria included loss of follow-up & dual biologic therapy. Statistical analyses were performed using Python.
Results: Of 202 CD patients who received RZB, 121 met criteria for analysis (Figure 1). Median diagnosis age was 27, 64% were female, and 79% Caucasian. Disease location varied; 46% had ileocolonic disease & remainder were evenly split (isolated ileal or colonic.) Most patients had prior biologic exposure. Thirty-nine (32%) patients had baseline arthralgia with minimal additional EIMs (< 3%) (Figure 2). In those with baseline arthralgia, median diagnosis age was higher (31 years vs 23.5, p = 0.009), & patients were seen more (17 times vs 12, p = 0.009). Of those with baseline arthralgia, 54% had improvement with RZB. This subgroup had less historic TNF- α exposure & lower baseline ESR (9 vs 19.5, p = 0.041). Thirteen patients without arthralgia developed symptoms on RZB & were older at diagnosis. Baseline patient demographics and markers of disease location / severity were similar between all groups.
Discussion: This retrospective study of CD patients on RZB found minimal baseline indicators of EIM response. Patients with baseline arthralgia were older at diagnosis, & of this subset, those with arthralgia improvement were less likely to have historic TNF-α therapy. Those who developed arthralgia after drug initiation were older at diagnosis. Demographics, disease classification, baseline disease severity, and inflammatory markers except ESR were not effective at predicting arthralgia response to RZB.
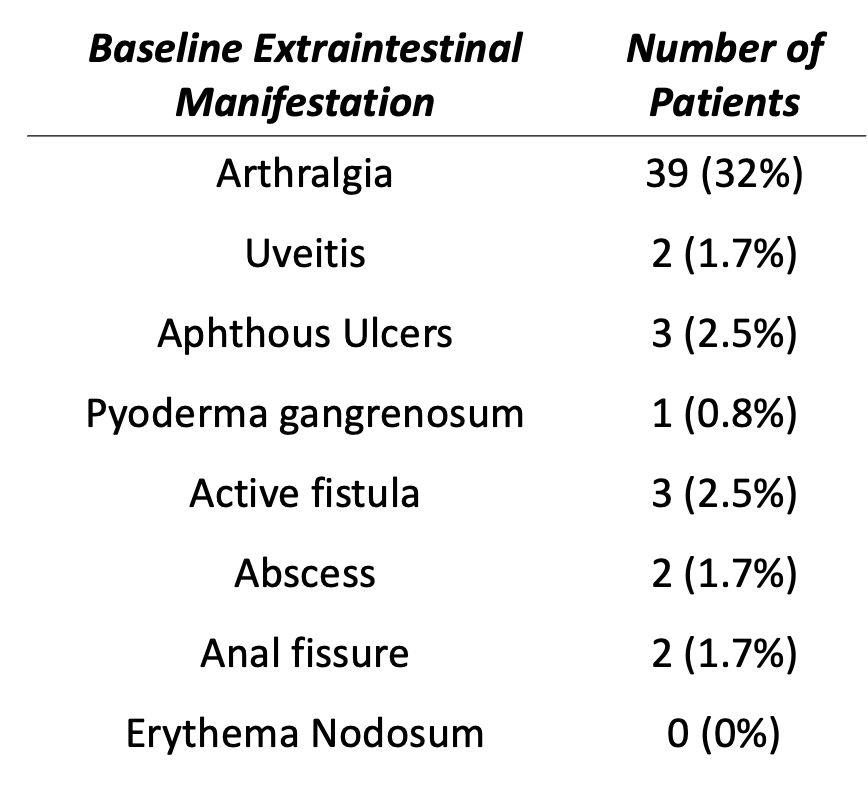
Figure: 1A: Comparison of Patient Characteristics and Risankizumab Response in Patients with and without Baseline Arthralgia
Abbreviations: Tumor Necrosis Factor (TNF), Janus Kinase (JAK), sphingosine-1-phosphate (S1P), Interleukin 23 (IL23), Erythrosite Sedimentation Rate (ESR), C-reactive Protein (CRP)
1B: Comparison of Patient Characteristics and Risankizumab Response in Patients with and without Interval Arthralgia Improvement
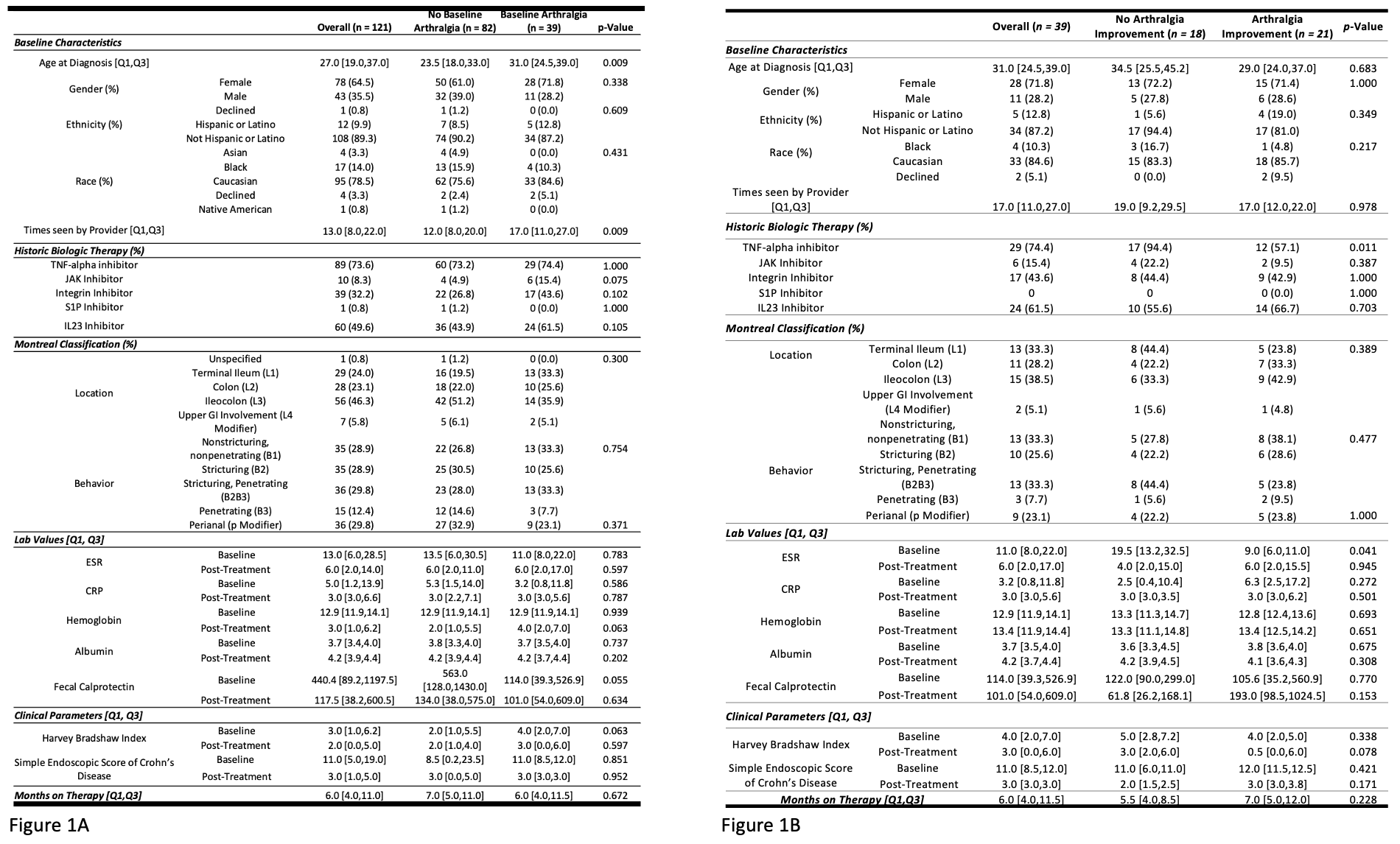
Figure: Figure 2: Baseline extraintestinal manifestations of Crohn’s Disease present in the full patient cohort.
Disclosures:
Hannah O'Neill indicated no relevant financial relationships.
Oscar Noble indicated no relevant financial relationships.
Poojasree Pasupuleti indicated no relevant financial relationships.
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Christopher Fan indicated no relevant financial relationships.
Hannah O'Neill, MD1, Oscar Noble, MD2, Poojasree Pasupuleti, MS2, Bincy Abraham, MD, MS, FACG3, Christopher Fan, MD4. P3273 - Effects of Risankizumab on the Extraintestinal Manifestations of Crohn's Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Houston Methodist-Weill Cornell Graduate School of Medical Sciences, Houston, TX; 2Houston Methodist Hospital, Division of Gastroenterology and Hepatology, Lynda K. and David M. Underwood Center for Digestive Health / Houston Methodist Research Institute, Department of Medicine, Houston, TX; 3Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX; 4Houston Methodist Hospital, Division of Gastroenterology and Hepatology, Lynda K. and David M. Underwood Center for Digestive Health / Houston Methodist Research Institute, Department of Medicine / Weill Cornell Medical College, Cornell University, Houston, TX
Introduction: Crohn’s disease (CD), characterized by chronic transmural inflammation of the GI tract, can lead to strictures & fistulas, obstruction, & cancer. Additionally, a wide range of debilitating extra-intestinal manifestations (EIMs) can occur. EIM response to CD treatment is variable & does not always coincide with IBD response. Biologic options for CD treatment now include risankizumab (RZB), an interleukin-23 inhibitor (IL-23i). This analysis aimed to assess RZB effects on EIMs & determine any baseline characteristics that predict response.
Methods: This retrospective, single center study included CD patients receiving RZB from 2022 to 2024. Baseline characteristics were examined to determine predictors of EIM response. Variables included demographics, markers of disease location / severity (Montreal Classification, fecal calprotectin, inflammatory markers), & prior medical therapies. Clinical parameters included Harvey-Bradshaw Index & Simple Endoscopic Score for CD. Exclusion criteria included loss of follow-up & dual biologic therapy. Statistical analyses were performed using Python.
Results: Of 202 CD patients who received RZB, 121 met criteria for analysis (Figure 1). Median diagnosis age was 27, 64% were female, and 79% Caucasian. Disease location varied; 46% had ileocolonic disease & remainder were evenly split (isolated ileal or colonic.) Most patients had prior biologic exposure. Thirty-nine (32%) patients had baseline arthralgia with minimal additional EIMs (< 3%) (Figure 2). In those with baseline arthralgia, median diagnosis age was higher (31 years vs 23.5, p = 0.009), & patients were seen more (17 times vs 12, p = 0.009). Of those with baseline arthralgia, 54% had improvement with RZB. This subgroup had less historic TNF- α exposure & lower baseline ESR (9 vs 19.5, p = 0.041). Thirteen patients without arthralgia developed symptoms on RZB & were older at diagnosis. Baseline patient demographics and markers of disease location / severity were similar between all groups.
Discussion: This retrospective study of CD patients on RZB found minimal baseline indicators of EIM response. Patients with baseline arthralgia were older at diagnosis, & of this subset, those with arthralgia improvement were less likely to have historic TNF-α therapy. Those who developed arthralgia after drug initiation were older at diagnosis. Demographics, disease classification, baseline disease severity, and inflammatory markers except ESR were not effective at predicting arthralgia response to RZB.

Figure: 1A: Comparison of Patient Characteristics and Risankizumab Response in Patients with and without Baseline Arthralgia
Abbreviations: Tumor Necrosis Factor (TNF), Janus Kinase (JAK), sphingosine-1-phosphate (S1P), Interleukin 23 (IL23), Erythrosite Sedimentation Rate (ESR), C-reactive Protein (CRP)
1B: Comparison of Patient Characteristics and Risankizumab Response in Patients with and without Interval Arthralgia Improvement

Figure: Figure 2: Baseline extraintestinal manifestations of Crohn’s Disease present in the full patient cohort.
Disclosures:
Hannah O'Neill indicated no relevant financial relationships.
Oscar Noble indicated no relevant financial relationships.
Poojasree Pasupuleti indicated no relevant financial relationships.
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Christopher Fan indicated no relevant financial relationships.
Hannah O'Neill, MD1, Oscar Noble, MD2, Poojasree Pasupuleti, MS2, Bincy Abraham, MD, MS, FACG3, Christopher Fan, MD4. P3273 - Effects of Risankizumab on the Extraintestinal Manifestations of Crohn's Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

