Monday Poster Session
Category: IBD
P3268 - Efficacy and Safety of Vedolizumab for Prevention of Postoperative Recurrence in Crohn’s Disease: A Systematic Review and Meta-Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Nouman Shafique, MD (he/him/his)
AdventHealth Orlando
Orlando, FL
Presenting Author(s)
Nouman Shafique, MD1, Nihal I. Khan, MD1, Tareq Alsaleh, MD2, Abdullah Javed, MBBS3, Shahzad Zafar, MD4, Osama Ijaz, MD5, Atta Ur Rehman, MBBS6, Ali Haider, MBBS7, Sheraz Ahmad Tariq, MBBS6, Adeena Shafique, MBBS8, Syed Hamaad Rahman, DO9, Iqra Shafique, MBBS10, Sobaan Taj, MD11, Abdul Wasay, MD12, Abu Hurairah, MD11, Babu P. Mohan, MD13
1AdventHealth Orlando, Orlando, FL; 2Department of Internal Medicine, Adventhealth Orlando, Orlando, FL; 3Allama Iqbal Medical College, Lahore, Punjab, Pakistan; 4Howard University Hospital, Washington, DC; 5SSM Health St. Mary's Hospital - St. Louis, Richmond Heights, MO; 6Nishtar Medical University, Multan, Punjab, Pakistan; 7Nishtar Medical University, Multan, Multan, Punjab, Pakistan; 8Aga Khan University, Karachi, Sindh, Pakistan; 9Methodist Dallas Medical Center, Dallas, TX; 10CMH Institute of Medical Sciences, Bahawalpur, Punjab, Pakistan; 11AdventHealth, Orlando, FL; 12University of Tennessee, Knoxville, TN; 13Orlando Gastroenterology PA, Orlando, FL
Introduction: Postoperative recurrence (POR) remains a significant challenge in Crohn’s disease (CD) patients with high-risk features. Although anti-TNF agents are currently the most validated biologic therapies in this setting, vedolizumab, a gut-selective integrin antagonist, has been studied in this clinical setting. However, its real-world efficacy and safety in preventing POR remain unclear. We conducted a single-arm proportional meta-analysis to evaluate rates of endoscopic, clinical, and biologic recurrence, as well as adverse events in CD patients receiving vedolizumab postoperatively.
Methods: We systematically searched MEDLINE, EMBASE, and Scopus from inception through May 2025 for studies assessing vedolizumab for prevention of POR in CD patients. Inclusion criteria were postoperative CD patients treated with vedolizumab with reported rates of clinical, endoscopic, or biologic recurrence. A total of 5 studies met inclusion criteria. We extracted pooled proportions of outcomes using a random-effects model. Definitions of recurrence followed each study’s protocol, including modified Rutgeerts scores for endoscopic POR, Harvey-Bradshaw Index or physician assessment for clinical POR, and C-reactive protein or fecal calprotectin for biologic POR.
Results: Across 5 studies, 187 patients were included with 43.8% females. Most patients had prior anti-TNF exposure and at least one high-risk feature for recurrence, including prior resections, penetrating disease, or active smoking. Baseline characteristics are summarized in Table 1; pooled outcomes are shown in Figure 1.
The pooled rate of clinical POR was 40.4% (95% CI: 18.7-62.0), endoscopic POR 43.1% (95% CI: 26.4-59.9), and biologic POR 67.4 %(95% CI: 56.3-78.5). Adverse events occurred in only 2.9% of patients (95% CI: 0.5-5.3).
Discussion: Vedolizumab shows moderate effectiveness in preventing postoperative recurrence in Crohn’s disease, with clinical and endoscopic recurrence near 40% and few adverse events. Controlled prospective trials are needed to further define its role and optimize postoperative management strategies in CD.
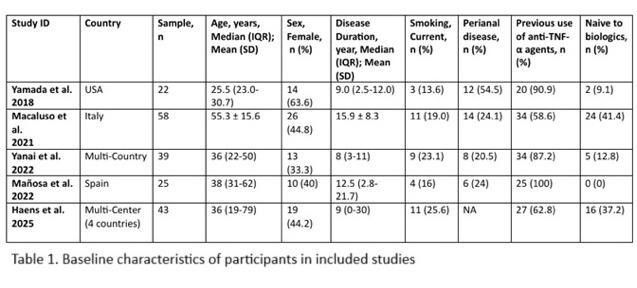
Figure: Table 1 showing Baseline Characteristics of all studies
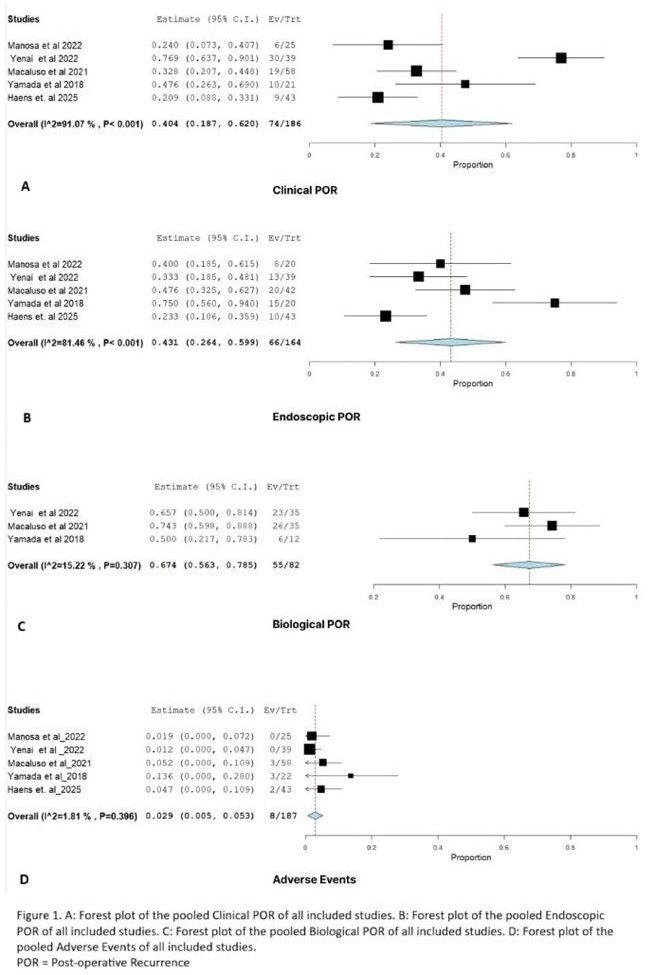
Figure: Forest Plots for Clinical, endoscopic, biological POR and adverse events
Disclosures:
Nouman Shafique indicated no relevant financial relationships.
Nihal Khan indicated no relevant financial relationships.
Tareq Alsaleh indicated no relevant financial relationships.
Abdullah Javed indicated no relevant financial relationships.
Shahzad Zafar indicated no relevant financial relationships.
Osama Ijaz indicated no relevant financial relationships.
Atta Ur Rehman indicated no relevant financial relationships.
Ali Haider indicated no relevant financial relationships.
Sheraz Ahmad Tariq indicated no relevant financial relationships.
Adeena Shafique indicated no relevant financial relationships.
Syed Hamaad Rahman indicated no relevant financial relationships.
Iqra Shafique indicated no relevant financial relationships.
Sobaan Taj indicated no relevant financial relationships.
Abdul Wasay indicated no relevant financial relationships.
Abu Hurairah indicated no relevant financial relationships.
Babu Mohan indicated no relevant financial relationships.
Nouman Shafique, MD1, Nihal I. Khan, MD1, Tareq Alsaleh, MD2, Abdullah Javed, MBBS3, Shahzad Zafar, MD4, Osama Ijaz, MD5, Atta Ur Rehman, MBBS6, Ali Haider, MBBS7, Sheraz Ahmad Tariq, MBBS6, Adeena Shafique, MBBS8, Syed Hamaad Rahman, DO9, Iqra Shafique, MBBS10, Sobaan Taj, MD11, Abdul Wasay, MD12, Abu Hurairah, MD11, Babu P. Mohan, MD13. P3268 - Efficacy and Safety of Vedolizumab for Prevention of Postoperative Recurrence in Crohn’s Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1AdventHealth Orlando, Orlando, FL; 2Department of Internal Medicine, Adventhealth Orlando, Orlando, FL; 3Allama Iqbal Medical College, Lahore, Punjab, Pakistan; 4Howard University Hospital, Washington, DC; 5SSM Health St. Mary's Hospital - St. Louis, Richmond Heights, MO; 6Nishtar Medical University, Multan, Punjab, Pakistan; 7Nishtar Medical University, Multan, Multan, Punjab, Pakistan; 8Aga Khan University, Karachi, Sindh, Pakistan; 9Methodist Dallas Medical Center, Dallas, TX; 10CMH Institute of Medical Sciences, Bahawalpur, Punjab, Pakistan; 11AdventHealth, Orlando, FL; 12University of Tennessee, Knoxville, TN; 13Orlando Gastroenterology PA, Orlando, FL
Introduction: Postoperative recurrence (POR) remains a significant challenge in Crohn’s disease (CD) patients with high-risk features. Although anti-TNF agents are currently the most validated biologic therapies in this setting, vedolizumab, a gut-selective integrin antagonist, has been studied in this clinical setting. However, its real-world efficacy and safety in preventing POR remain unclear. We conducted a single-arm proportional meta-analysis to evaluate rates of endoscopic, clinical, and biologic recurrence, as well as adverse events in CD patients receiving vedolizumab postoperatively.
Methods: We systematically searched MEDLINE, EMBASE, and Scopus from inception through May 2025 for studies assessing vedolizumab for prevention of POR in CD patients. Inclusion criteria were postoperative CD patients treated with vedolizumab with reported rates of clinical, endoscopic, or biologic recurrence. A total of 5 studies met inclusion criteria. We extracted pooled proportions of outcomes using a random-effects model. Definitions of recurrence followed each study’s protocol, including modified Rutgeerts scores for endoscopic POR, Harvey-Bradshaw Index or physician assessment for clinical POR, and C-reactive protein or fecal calprotectin for biologic POR.
Results: Across 5 studies, 187 patients were included with 43.8% females. Most patients had prior anti-TNF exposure and at least one high-risk feature for recurrence, including prior resections, penetrating disease, or active smoking. Baseline characteristics are summarized in Table 1; pooled outcomes are shown in Figure 1.
The pooled rate of clinical POR was 40.4% (95% CI: 18.7-62.0), endoscopic POR 43.1% (95% CI: 26.4-59.9), and biologic POR 67.4 %(95% CI: 56.3-78.5). Adverse events occurred in only 2.9% of patients (95% CI: 0.5-5.3).
Discussion: Vedolizumab shows moderate effectiveness in preventing postoperative recurrence in Crohn’s disease, with clinical and endoscopic recurrence near 40% and few adverse events. Controlled prospective trials are needed to further define its role and optimize postoperative management strategies in CD.

Figure: Table 1 showing Baseline Characteristics of all studies

Figure: Forest Plots for Clinical, endoscopic, biological POR and adverse events
Disclosures:
Nouman Shafique indicated no relevant financial relationships.
Nihal Khan indicated no relevant financial relationships.
Tareq Alsaleh indicated no relevant financial relationships.
Abdullah Javed indicated no relevant financial relationships.
Shahzad Zafar indicated no relevant financial relationships.
Osama Ijaz indicated no relevant financial relationships.
Atta Ur Rehman indicated no relevant financial relationships.
Ali Haider indicated no relevant financial relationships.
Sheraz Ahmad Tariq indicated no relevant financial relationships.
Adeena Shafique indicated no relevant financial relationships.
Syed Hamaad Rahman indicated no relevant financial relationships.
Iqra Shafique indicated no relevant financial relationships.
Sobaan Taj indicated no relevant financial relationships.
Abdul Wasay indicated no relevant financial relationships.
Abu Hurairah indicated no relevant financial relationships.
Babu Mohan indicated no relevant financial relationships.
Nouman Shafique, MD1, Nihal I. Khan, MD1, Tareq Alsaleh, MD2, Abdullah Javed, MBBS3, Shahzad Zafar, MD4, Osama Ijaz, MD5, Atta Ur Rehman, MBBS6, Ali Haider, MBBS7, Sheraz Ahmad Tariq, MBBS6, Adeena Shafique, MBBS8, Syed Hamaad Rahman, DO9, Iqra Shafique, MBBS10, Sobaan Taj, MD11, Abdul Wasay, MD12, Abu Hurairah, MD11, Babu P. Mohan, MD13. P3268 - Efficacy and Safety of Vedolizumab for Prevention of Postoperative Recurrence in Crohn’s Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
