Monday Poster Session
Category: IBD
P3267 - Real-World Comparison of Every 6-week and Every 4-Week Ustekinumab Dose Escalation in Patients With Inflammatory Bowel Disease
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- AG
Aaron Goffinet, MD
University of Chicago Medical Center
Chicago, IL
Presenting Author(s)
Award: ACG Presidential Poster Award
Aaron Goffinet, MD1, David B. Cao, MD2, Allison Liu, MD, BA3, David Choi, PharmD4, Russell D. Cohen, MD2, Sushila Dalal, MD5, Noa Krugliak Cleveland, MD6, Joel Pekow, MD6, David T. Rubin, MD7, Tenzin Choden, MD5
1University of Chicago Medical Center, Chicago, IL; 2University of Chicago Medicine, Chicago, IL; 3University of California San Francisco, San Francisco, CA; 4University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 5University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 6University of Chicago, Chicago, IL; 7University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL
Introduction: Several studies have suggested that dose escalation of ustekinumab (UST) from 90 mg subcutaneous every 8 weeks (q8) to every 4 weeks (q4) or 6 weeks (q6) is effective for patients with inflammatory bowel disease (IBD) who do not obtain clinical or biochemical remission, but the decision to escalate to q6 or to q4 week dosing is non-standardized. We aimed to evaluate outcomes in patients with IBD with UST escalation to q6 and q4.
Methods: This is a retrospective analysis of patients who underwent UST escalation at our tertiary center between 8/15/2019-4/15/2024. We collected demographics, treatment exposures, steroid use, clinical activity scores, endoscopy findings, biomarkers, and duration of therapy. Treatment success was defined using clinical and objective measures. Statistical comparisons between q6 and q4 week dosing outcomes were performed using Fisher’s Exact Test, Welch’s t-test, and Wilcoxon rank-sum test.
Results: 102 patients (71 CD, 29 UC) underwent UST weight based IV loading and maintenance dosing of 90 mg SC q8, but subsequently escalated to q6 (57, 55.9%) or q4 (45, 44.1%). Demographics, disease characteristics, and median time to outcomes are reported in Table 1.
In the q6 group, 52.6% (30/57) remained on therapy for ≥12 months compared to 75.6% (34/45) in the q4 group (p = 0.02). In the q6 group, 17.5% (10/57) transitioned to alternative therapy within 4 months compared to 11.1% (5/45) in the q4 group (p= 0.41). Mean duration of therapy for q6 was significantly shorter than q4 (13.2 vs 20.6 months (p-value= 0.003)).
In the q6 group, 49.1% (28/57) achieved steroid free clinical remission (HBI ≤4, SCCAI ≤2, or provider assessment if not documented), versus 71.1% (32/45) in the q4 group (p = 0.03). In the q6 group, 66.7% (12/18) of patients and 76% (19/25) in the q4 group demonstrated mild or no inflammation on post-escalation endoscopic evaluation (p = 0.51). Of patients with elevated CRP pre-escalation, the q6 group had 4/16 patients achieve CRP normalization post-escalation compared to 5/13 patients in the q4 group (p= 0.69).
Discussion: In this real-world cohort of patients with IBD undergoing UST dose interval reduction, escalation to q4 was associated with significantly longer therapy duration and higher rates of steroid-free clinical remission compared to q6. These findings support considering q4 escalation as a preferred strategy for patients requiring UST optimization prior to switching to alternative therapies.
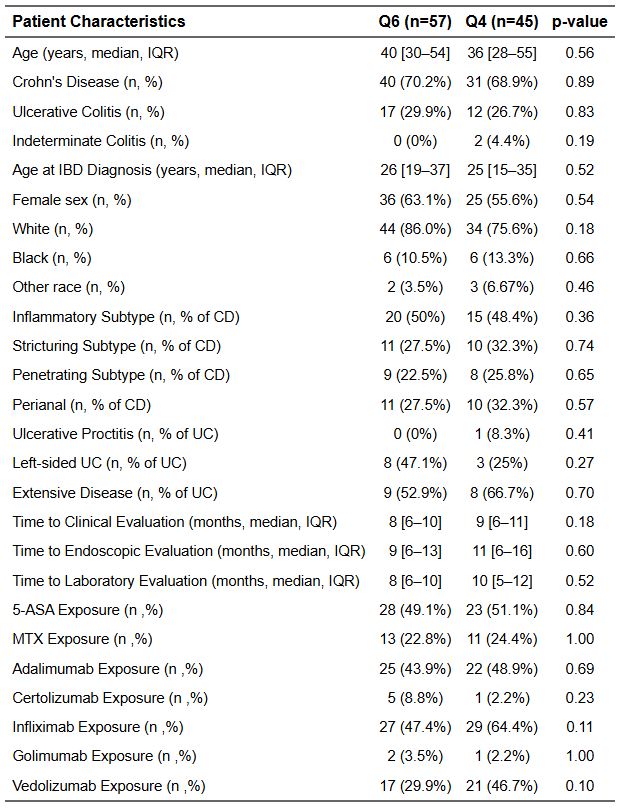
Figure: Table 1: Patient characteristics between every 6 and every 4 week escalation groups.
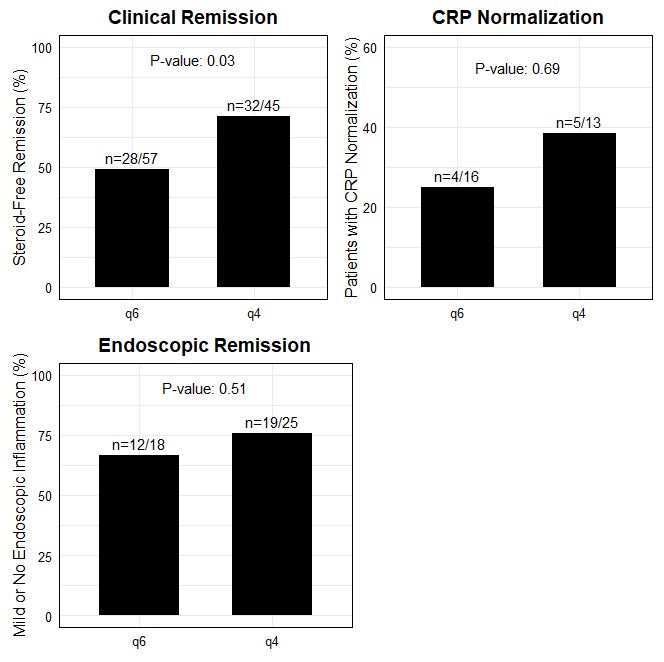
Figure: Figure 1: Clinical, Endoscopic, and Biomarker outcomes compared between q6 and q4 escalation groups. A significantly higher proportion of patients achieved steroid free clinical remission in the q4 group compared to q6; however, no differences were seen between endoscopic or biomarker outcomes.
Disclosures:
Aaron Goffinet indicated no relevant financial relationships.
David Cao indicated no relevant financial relationships.
Allison Liu indicated no relevant financial relationships.
David Choi: Abbvie – Advisory Committee/Board Member, Speakers Bureau. Boehringer – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Johnson and Johnso – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member.
Russell Cohen: Abbvie – Consultant, Speakers Bureau. Bausch Health – Consultant. BMS – Consultant. Eli lilly – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. Johnson & Johnson – Consultant. Pfizer – Consultant. Takeda – Consultant.
Sushila Dalal: Abbvie – Speakers Bureau.
Noa Krugliak Cleveland: Johnson & Johnson – Consultant. NueroLogica – Consultant.
Joel Pekow: Abbvie – Stock-publicly held company(excluding mutual/index funds). CVS Health – Consultant. Eli Lilly – Stock-publicly held company(excluding mutual/index funds). Johnson and Johnson – Stock-publicly held company(excluding mutual/index funds). Pfizer – Stock-publicly held company(excluding mutual/index funds).
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Tenzin Choden indicated no relevant financial relationships.
Aaron Goffinet, MD1, David B. Cao, MD2, Allison Liu, MD, BA3, David Choi, PharmD4, Russell D. Cohen, MD2, Sushila Dalal, MD5, Noa Krugliak Cleveland, MD6, Joel Pekow, MD6, David T. Rubin, MD7, Tenzin Choden, MD5. P3267 - Real-World Comparison of Every 6-week and Every 4-Week Ustekinumab Dose Escalation in Patients With Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Aaron Goffinet, MD1, David B. Cao, MD2, Allison Liu, MD, BA3, David Choi, PharmD4, Russell D. Cohen, MD2, Sushila Dalal, MD5, Noa Krugliak Cleveland, MD6, Joel Pekow, MD6, David T. Rubin, MD7, Tenzin Choden, MD5
1University of Chicago Medical Center, Chicago, IL; 2University of Chicago Medicine, Chicago, IL; 3University of California San Francisco, San Francisco, CA; 4University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 5University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 6University of Chicago, Chicago, IL; 7University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL
Introduction: Several studies have suggested that dose escalation of ustekinumab (UST) from 90 mg subcutaneous every 8 weeks (q8) to every 4 weeks (q4) or 6 weeks (q6) is effective for patients with inflammatory bowel disease (IBD) who do not obtain clinical or biochemical remission, but the decision to escalate to q6 or to q4 week dosing is non-standardized. We aimed to evaluate outcomes in patients with IBD with UST escalation to q6 and q4.
Methods: This is a retrospective analysis of patients who underwent UST escalation at our tertiary center between 8/15/2019-4/15/2024. We collected demographics, treatment exposures, steroid use, clinical activity scores, endoscopy findings, biomarkers, and duration of therapy. Treatment success was defined using clinical and objective measures. Statistical comparisons between q6 and q4 week dosing outcomes were performed using Fisher’s Exact Test, Welch’s t-test, and Wilcoxon rank-sum test.
Results: 102 patients (71 CD, 29 UC) underwent UST weight based IV loading and maintenance dosing of 90 mg SC q8, but subsequently escalated to q6 (57, 55.9%) or q4 (45, 44.1%). Demographics, disease characteristics, and median time to outcomes are reported in Table 1.
In the q6 group, 52.6% (30/57) remained on therapy for ≥12 months compared to 75.6% (34/45) in the q4 group (p = 0.02). In the q6 group, 17.5% (10/57) transitioned to alternative therapy within 4 months compared to 11.1% (5/45) in the q4 group (p= 0.41). Mean duration of therapy for q6 was significantly shorter than q4 (13.2 vs 20.6 months (p-value= 0.003)).
In the q6 group, 49.1% (28/57) achieved steroid free clinical remission (HBI ≤4, SCCAI ≤2, or provider assessment if not documented), versus 71.1% (32/45) in the q4 group (p = 0.03). In the q6 group, 66.7% (12/18) of patients and 76% (19/25) in the q4 group demonstrated mild or no inflammation on post-escalation endoscopic evaluation (p = 0.51). Of patients with elevated CRP pre-escalation, the q6 group had 4/16 patients achieve CRP normalization post-escalation compared to 5/13 patients in the q4 group (p= 0.69).
Discussion: In this real-world cohort of patients with IBD undergoing UST dose interval reduction, escalation to q4 was associated with significantly longer therapy duration and higher rates of steroid-free clinical remission compared to q6. These findings support considering q4 escalation as a preferred strategy for patients requiring UST optimization prior to switching to alternative therapies.

Figure: Table 1: Patient characteristics between every 6 and every 4 week escalation groups.

Figure: Figure 1: Clinical, Endoscopic, and Biomarker outcomes compared between q6 and q4 escalation groups. A significantly higher proportion of patients achieved steroid free clinical remission in the q4 group compared to q6; however, no differences were seen between endoscopic or biomarker outcomes.
Disclosures:
Aaron Goffinet indicated no relevant financial relationships.
David Cao indicated no relevant financial relationships.
Allison Liu indicated no relevant financial relationships.
David Choi: Abbvie – Advisory Committee/Board Member, Speakers Bureau. Boehringer – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Johnson and Johnso – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member.
Russell Cohen: Abbvie – Consultant, Speakers Bureau. Bausch Health – Consultant. BMS – Consultant. Eli lilly – Consultant. Genentech – Consultant. Gilead Sciences – Consultant. Johnson & Johnson – Consultant. Pfizer – Consultant. Takeda – Consultant.
Sushila Dalal: Abbvie – Speakers Bureau.
Noa Krugliak Cleveland: Johnson & Johnson – Consultant. NueroLogica – Consultant.
Joel Pekow: Abbvie – Stock-publicly held company(excluding mutual/index funds). CVS Health – Consultant. Eli Lilly – Stock-publicly held company(excluding mutual/index funds). Johnson and Johnson – Stock-publicly held company(excluding mutual/index funds). Pfizer – Stock-publicly held company(excluding mutual/index funds).
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Tenzin Choden indicated no relevant financial relationships.
Aaron Goffinet, MD1, David B. Cao, MD2, Allison Liu, MD, BA3, David Choi, PharmD4, Russell D. Cohen, MD2, Sushila Dalal, MD5, Noa Krugliak Cleveland, MD6, Joel Pekow, MD6, David T. Rubin, MD7, Tenzin Choden, MD5. P3267 - Real-World Comparison of Every 6-week and Every 4-Week Ustekinumab Dose Escalation in Patients With Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

