Monday Poster Session
Category: IBD
P3261 - Impact of Glucagon-Like Peptide-1 Receptor Agonists on Inflammatory Bowel Disease in Patients With and Without Type 2 Diabetes Mellitus
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Matthew Still, DO
University of Cincinnati Medical Center
Cincinnati, OH
Presenting Author(s)
Matthew Still, DO1, Titas Bera, MD1, Wei-Wen Hsu, PhD2, Susan Kais, MD1
1University of Cincinnati Medical Center, Cincinnati, OH; 2University of Cincinnati, Cincinnati, OH
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are established therapies for type 2 diabetes mellitus (T2DM) and obesity. Beyond their metabolic benefits, they also exhibit anti-inflammatory and immunomodulatory effects, raising interest in their potential use in inflammatory diseases. Previous retrospective studies have suggested possible benefits of GLP-1RA use in patients with T2DM and inflammatory bowel disease (IBD). With the expanding use of GLP-1RAs in non-diabetic populations, there is an increasing opportunity to investigate their effects in chronic inflammatory conditions. Using the TriNetX data network, this retrospective study aimed to assess the outcomes associated with GLP-1RA use in patients with IBD, both with and without T2DM.
Methods: Adults (aged 18–80 years) with Crohn’s disease or ulcerative colitis identified in the TriNetX database (defined via International Classification of Diseases (ICD) and IBD medication RxNorm codes) were stratified into cohorts based on T2DM status (ICD codes or HbA1c) and documented GLP-1RA prescription (RxNorm codes) for at least six months. Propensity score matching (PSM, 1:1) was used to adjust for age, sex, BMI, race, smoking, alcohol use, and HbA1c. Outcomes included rates of hospitalizations, gastrointestinal surgeries, flares (defined by methylprednisolone use), mortality, malignancy incidence, and levels of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Risk ratios and 95% confidence intervals were calculated. Means ± standard deviations were reported for lab results. Outcomes were assessed over three years after GLP-1RA prescription or matched index date within the study period (2015-2025).
Results: Matched IBD cohorts included 2,372 T2DM and 1,244 non-T2DM pairs. Compared to non-users, GLP-1RA users had significantly lower risks of surgeries, hospitalizations, and mortality regardless of diabetes status (Table 1). GLP-1RAs were also associated with lower CRP and ESR levels (Table 2). Methylprednisolone use was lower among GLP-1RA users with T2DM, but was not statistically significant in the non-T2DM group. No significant differences in malignancy incidence were observed in either group.
Discussion: GLP-1RAs in patients with IBD were associated with reduced rates of surgeries, hospitalizations, mortality, and lower CRP and ESR levels, regardless of T2DM status. These findings support further investigation of GLP-1RAs as potential primary or adjunctive therapies for IBD.
Condensed by AI; all edits reviewed.
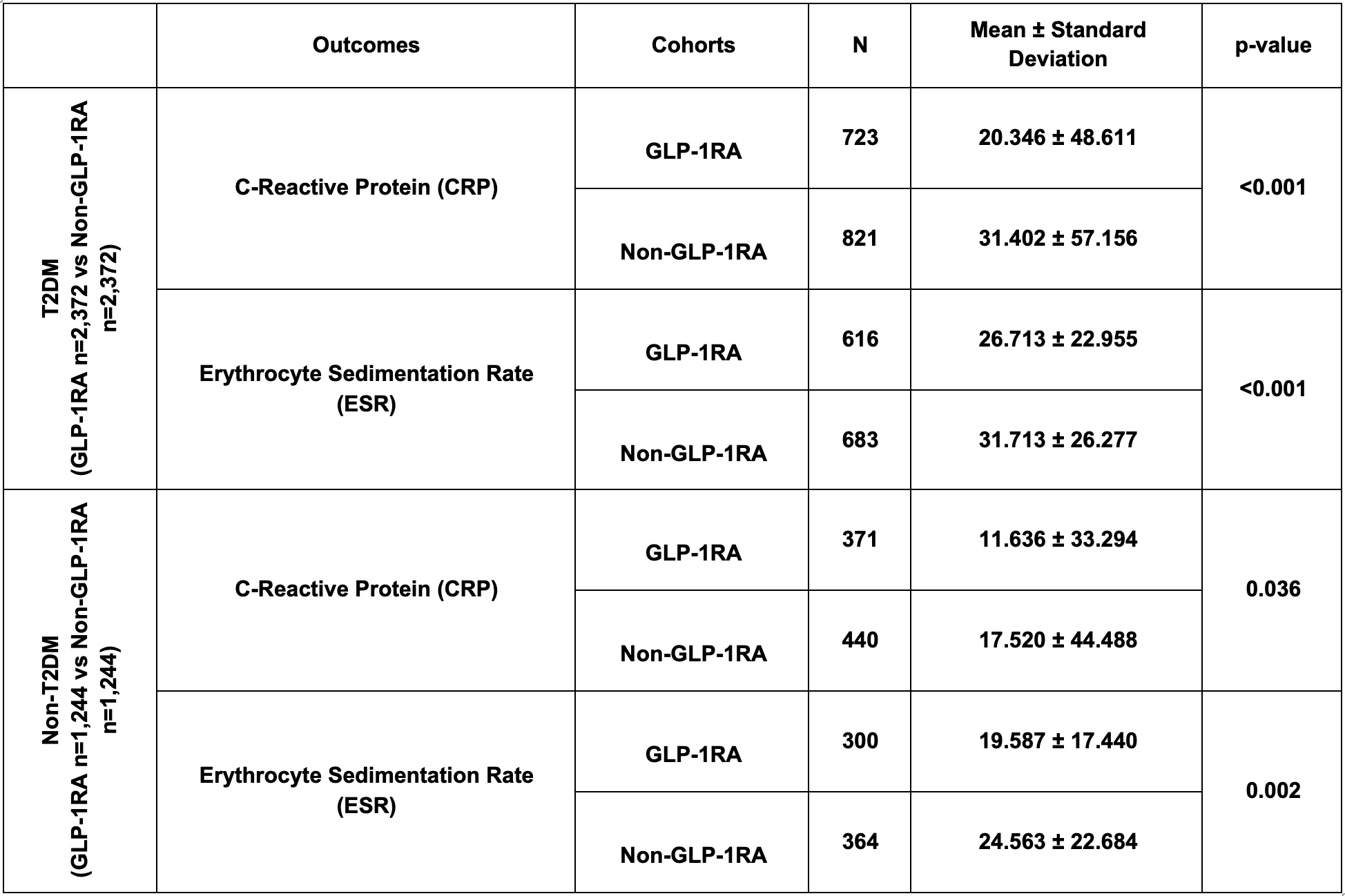
Figure: Table 1: Outcomes in patients in GLP-1RA & Non-GLP-1RA cohorts with and without T2DM after propensity score matching with relative risk.
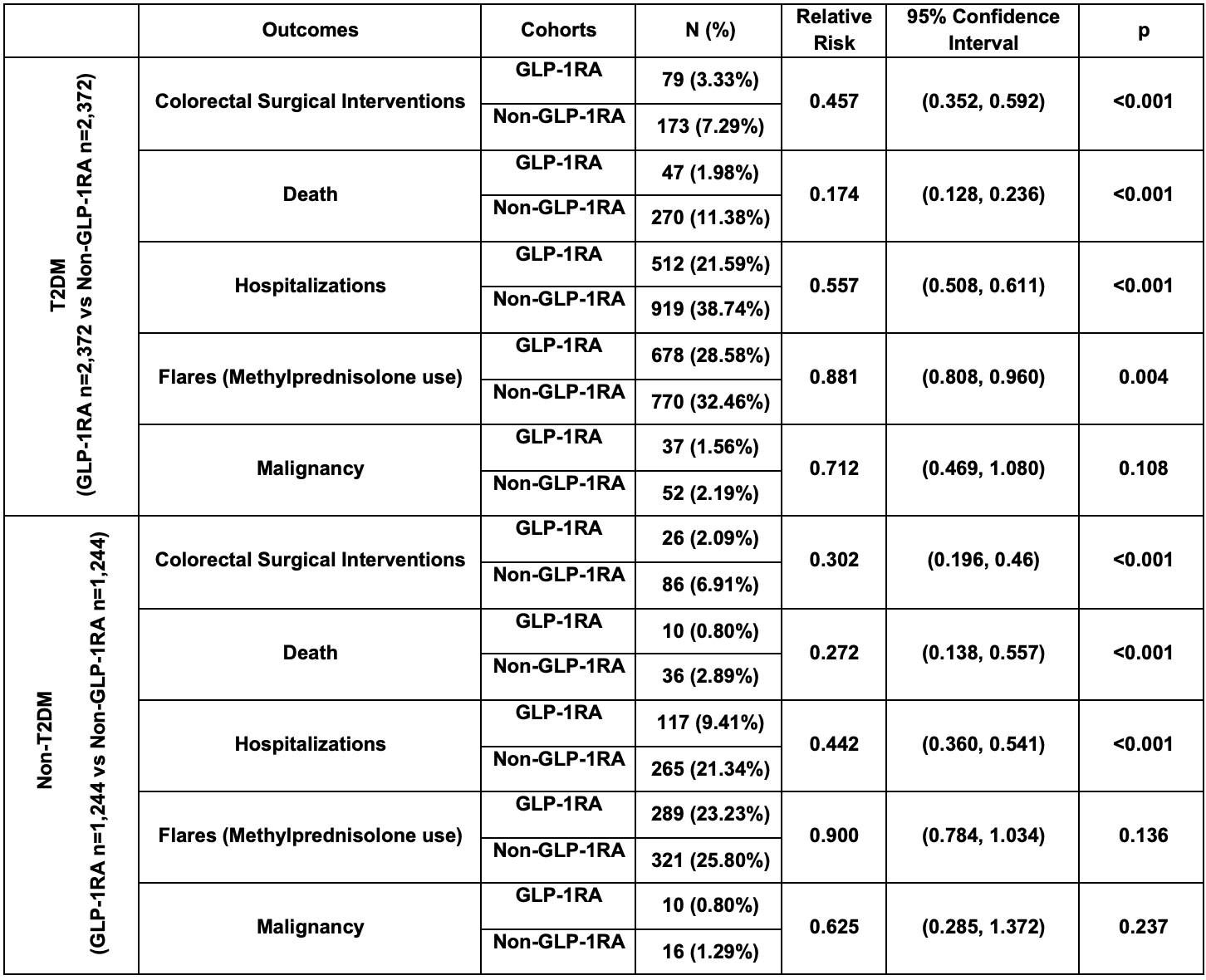
Figure: Table 2: Inflammatory markers in patients in GLP-1RA & Non-GLP-1RA cohorts with and without T2DM after propensity score matching.
Disclosures:
Matthew Still indicated no relevant financial relationships.
Titas Bera indicated no relevant financial relationships.
Wei-Wen Hsu indicated no relevant financial relationships.
Susan Kais: Abbvie – Advisory Committee/Board Member, Marketing, Speakers Bureau. BMS – Advisory Committee/Board Member, Speakers Bureau. Jansen – Speakers Bureau.
Matthew Still, DO1, Titas Bera, MD1, Wei-Wen Hsu, PhD2, Susan Kais, MD1. P3261 - Impact of Glucagon-Like Peptide-1 Receptor Agonists on Inflammatory Bowel Disease in Patients With and Without Type 2 Diabetes Mellitus, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Cincinnati Medical Center, Cincinnati, OH; 2University of Cincinnati, Cincinnati, OH
Introduction: Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are established therapies for type 2 diabetes mellitus (T2DM) and obesity. Beyond their metabolic benefits, they also exhibit anti-inflammatory and immunomodulatory effects, raising interest in their potential use in inflammatory diseases. Previous retrospective studies have suggested possible benefits of GLP-1RA use in patients with T2DM and inflammatory bowel disease (IBD). With the expanding use of GLP-1RAs in non-diabetic populations, there is an increasing opportunity to investigate their effects in chronic inflammatory conditions. Using the TriNetX data network, this retrospective study aimed to assess the outcomes associated with GLP-1RA use in patients with IBD, both with and without T2DM.
Methods: Adults (aged 18–80 years) with Crohn’s disease or ulcerative colitis identified in the TriNetX database (defined via International Classification of Diseases (ICD) and IBD medication RxNorm codes) were stratified into cohorts based on T2DM status (ICD codes or HbA1c) and documented GLP-1RA prescription (RxNorm codes) for at least six months. Propensity score matching (PSM, 1:1) was used to adjust for age, sex, BMI, race, smoking, alcohol use, and HbA1c. Outcomes included rates of hospitalizations, gastrointestinal surgeries, flares (defined by methylprednisolone use), mortality, malignancy incidence, and levels of C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Risk ratios and 95% confidence intervals were calculated. Means ± standard deviations were reported for lab results. Outcomes were assessed over three years after GLP-1RA prescription or matched index date within the study period (2015-2025).
Results: Matched IBD cohorts included 2,372 T2DM and 1,244 non-T2DM pairs. Compared to non-users, GLP-1RA users had significantly lower risks of surgeries, hospitalizations, and mortality regardless of diabetes status (Table 1). GLP-1RAs were also associated with lower CRP and ESR levels (Table 2). Methylprednisolone use was lower among GLP-1RA users with T2DM, but was not statistically significant in the non-T2DM group. No significant differences in malignancy incidence were observed in either group.
Discussion: GLP-1RAs in patients with IBD were associated with reduced rates of surgeries, hospitalizations, mortality, and lower CRP and ESR levels, regardless of T2DM status. These findings support further investigation of GLP-1RAs as potential primary or adjunctive therapies for IBD.
Condensed by AI; all edits reviewed.

Figure: Table 1: Outcomes in patients in GLP-1RA & Non-GLP-1RA cohorts with and without T2DM after propensity score matching with relative risk.

Figure: Table 2: Inflammatory markers in patients in GLP-1RA & Non-GLP-1RA cohorts with and without T2DM after propensity score matching.
Disclosures:
Matthew Still indicated no relevant financial relationships.
Titas Bera indicated no relevant financial relationships.
Wei-Wen Hsu indicated no relevant financial relationships.
Susan Kais: Abbvie – Advisory Committee/Board Member, Marketing, Speakers Bureau. BMS – Advisory Committee/Board Member, Speakers Bureau. Jansen – Speakers Bureau.
Matthew Still, DO1, Titas Bera, MD1, Wei-Wen Hsu, PhD2, Susan Kais, MD1. P3261 - Impact of Glucagon-Like Peptide-1 Receptor Agonists on Inflammatory Bowel Disease in Patients With and Without Type 2 Diabetes Mellitus, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
