Monday Poster Session
Category: IBD
P3252 - Mirikizumab Crohn's Disease VIVID-1 Study Placebo Response Differences Between the United States Labeling and Primary Manuscript Due to Methodological Differences in Handling Participants Randomized to Placebo
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Miguel Regueiro, MD
Cleveland Clinic
Cleveland, OH
Presenting Author(s)
Bruce E. Sands, MD, MS, FACG1, Jessica R.. Allegretti, MD, MPH2, Miguel Regueiro, MD3, David B.. Clemow, PhD4, Deborah A.. Fisher, MD5, Guanglei Yu, PhD5, Richard E.. Moses, DO, JD4, Gary R.. Lichtenstein, MD, FACG6
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 3Cleveland Clinic, Cleveland, OH; 4Eli Lilly and Company, Indianapolis, IN; 5Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 6University of Pennsylvania, Philadelphia, PA
Introduction: Different methods for handling placebo (PBO) response in a treat-through design study, where patients receiving PBO are switched to active treatment after induction, can lead to differences in reported PBO response rates. Mirikizumab (MIRI) effect size has been reported differently in publications1 compared with the United States Package Insert (USPI)2. We discuss these different methodologies and their impact on determining MIRI’s effect size.
Methods: For VIVID-1 publications, inclusion criteria for the primary analysis set (PAS) population were a baseline unweighted daily average frequency of loose/watery stool ≥4 and/or unweighted daily average abdominal pain ≥2 and a centrally read Simple Endoscopic Score for Crohn’s Disease (SES-CD) score ≥7 or ≥4 for patients with isolated ileal disease. Week (W) 12 clinical response was determined by Patient Reported Outcome (PRO). Nonresponder imputation (NRI) was used. PBO patients who did not achieve PRO clinical response at W12 were switched to MIRI and analyzed as NRs for all W52 efficacy outcomes (PBO/PBO W12 Observation Carried Forward method) (Figure 1). In the USPI, PBO patients switched to MIRI at W12 were analyzed as PBO patients with their observed W52 results used for all W52 efficacy endpoints despite having received MIRI for 40 weeks (PBO/PBO + PBO/MIRI Observed method). To assess the impact of the two methods on MIRI effect size, we examined efficacy in the USPI population using both methods.
Results: For the endpoints included, W52 PBO rates were 5-17% greater for the observed method compared with the observation carried forward method, thereby changing MIRI effect size by the same amount (Figure 2). Across the four W52 endpoints, a range of 48-69% of the W52 PBO response rate for the observed method was driven by patients with 40 weeks treatment on MIRI.
Discussion: PBO rate differences between the MIRI USPI and published VIVID-1 analyses are due to different methodologic approaches to calculating treatment response. Published greater differences in the treatment effect between MIRI and PBO over 52 weeks included MIRI-treated patients but as NRs (40 weeks of MIRI treatment) in the PBO response rate, whereas these patients were included with observed data in the method used for the USPI.
References:
1. Ferrante et al., The Lancet 2024; 404:2423-36.
2. OMVOH (Mirikizumab-mrkz) [US PI]. Indianapolis, IN, USA: Eli Lilly and Company, 2025.
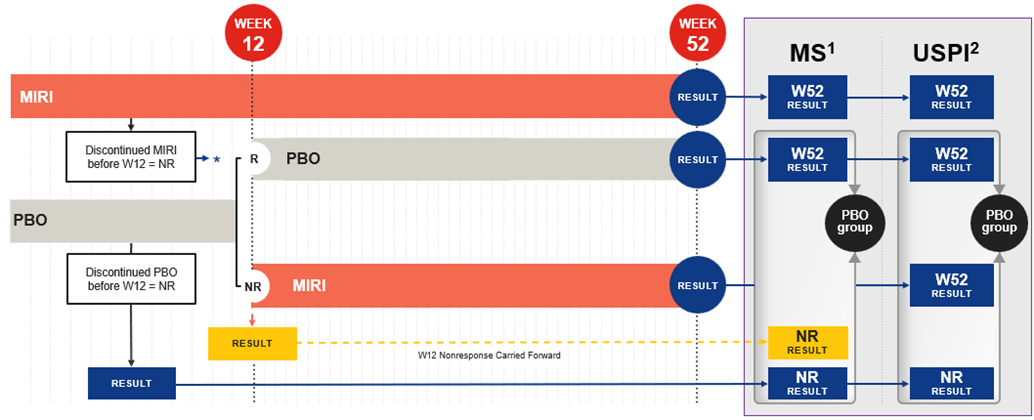
Figure: Figure 1. Methods of handling placebo in the VIVID-1 study manuscript and the USPI: Graphical comparison.1,2.
*NR result counted for MIRI result both in MS and USPI. Note: For the USPI, the PAS population inclusion criteria were a baseline Crohn’s Disease Activity Index (CDAI) total score ≥220 (1 clinical site was excluded); the SES-CD criteria were unchanged. This resulted in 31 fewer PBO and 68 fewer MIRI patients. Abbreviations: MIRI=Mirikizumab-mrkz; MS=Manuscript; NR=Nonresponders; PBO=Placebo; R=Responders; USPI=United States Package Insert; W=Week. 1. Ferrante et al., The Lancet 2024; 404:2423-36. 2. OMVOH (Mirikizumab-mrkz) [USPI]. Indianapolis, IN, USA: Eli Lilly and Company, 2025.
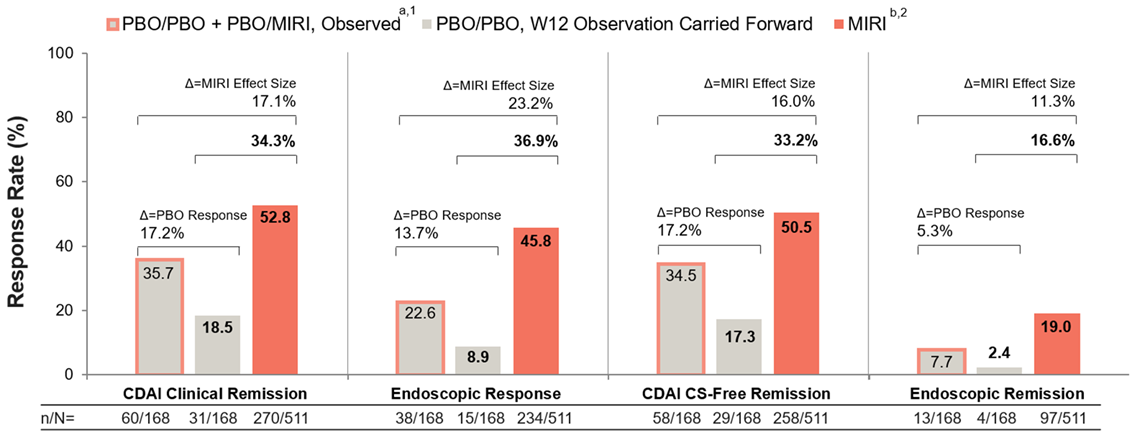
Figure: Figure 2. Efficacy at week 52 by placebo-handling method (VIVID-1 USPI population; NRI): Impact on mirikizumab effect size.
aThis includes all 168 patients randomized to PBO at baseline. Of those, 67 (40%) patients who did not achieve clinical response by PRO at W12 were switched to treatment with MIRI (designated as PBO/MIRI) and their efficacy data are included here with the remaining patients randomized to PBO who did not receive MIRI (designated as PBO). bFollowing MIRI 900 mg as an IV infusion at Week 0, Week 4, and Week 8, patients received MIRI 300 mg as a SC injection at W12 and every 4 weeks thereafter for up to an additional 40 weeks. CDAI=Crohn's Disease Activity Index; CS=Corticosteroid; IV=Intravenous; MIRI=Mirikizumab-mrkz; N=Number of Patients in the Analysis Population; n=Number of Patients in the Specified Category; NRI=Nonresponder Imputation; PBO=Placebo; PRO=Patient-Reported Outcome; SC=Subcutaneous; USPI=United States Package Insert; W=Week. 1. OMVOH (Mirikizumab-mrkz) [USPI]. Indianapolis, IN, USA: Eli Lilly and Company, 2025. 2. Ferrante et al., The Lancet 2024; 404:2423-36.
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Miguel Regueiro: AbbVie – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. BMS – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim Pharmaceuticals Inc. – Advisory Committee/Board Member, Consultant. Celegene – Advisory Committee/Board Member, Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Prometheus – Advisory Committee/Board Member, Consultant. Salix – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. UCB – Advisory Committee/Board Member, Consultant.
David Clemow: Eli Lilly and Company – Employee, Stock Options.
Deborah Fisher: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Richard Moses: Eli Lilly and Company – Employee, Stock Options.
Gary Lichtenstein: Abbvie – Consultant. American College of Gastroenterology – honorarium as associate editor of American Journal of Gastroenterology. Celgene – Consultant, Grant/Research Support. CellCeutrix – Consultant. Clinical Advances in Gastroenterology – Honorarium - Associate Editor. Eli Lilly – Consultant, DSMB. Ferring – Consultant. Gastroenterology and Hepatology [Gastro-Hep Communications] – Honorarium - Editor. Gilead – Consultant. Johnson & Johnson – Consultant. Luitpold/American Regent – Consultant, Honorarium - CME programs. McMahon Publishing – Honorarium - Author. Merck – Consultant, Honorarium - CME programs. Orthobiotech – Consultant, Grant/Research Support. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Romark – Consultant, Honorarium - CME programs. Salix/Valeant – Consultant, Grant/Research Support. Shire – Consultant, Grant/Research Support. SLACK – Book royalty. Springer – Honorarium - Editor. Takeda – Consultant, Funding to institution [IBD fellow education]. UCB – Consultant, Grant/Research Support. Up-To-Date – Honorarium - Author. Vindico – CME. Virgo – Consultant, Stock Options.
Bruce E. Sands, MD, MS, FACG1, Jessica R.. Allegretti, MD, MPH2, Miguel Regueiro, MD3, David B.. Clemow, PhD4, Deborah A.. Fisher, MD5, Guanglei Yu, PhD5, Richard E.. Moses, DO, JD4, Gary R.. Lichtenstein, MD, FACG6. P3252 - Mirikizumab Crohn's Disease VIVID-1 Study Placebo Response Differences Between the United States Labeling and Primary Manuscript Due to Methodological Differences in Handling Participants Randomized to Placebo, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 3Cleveland Clinic, Cleveland, OH; 4Eli Lilly and Company, Indianapolis, IN; 5Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 6University of Pennsylvania, Philadelphia, PA
Introduction: Different methods for handling placebo (PBO) response in a treat-through design study, where patients receiving PBO are switched to active treatment after induction, can lead to differences in reported PBO response rates. Mirikizumab (MIRI) effect size has been reported differently in publications1 compared with the United States Package Insert (USPI)2. We discuss these different methodologies and their impact on determining MIRI’s effect size.
Methods: For VIVID-1 publications, inclusion criteria for the primary analysis set (PAS) population were a baseline unweighted daily average frequency of loose/watery stool ≥4 and/or unweighted daily average abdominal pain ≥2 and a centrally read Simple Endoscopic Score for Crohn’s Disease (SES-CD) score ≥7 or ≥4 for patients with isolated ileal disease. Week (W) 12 clinical response was determined by Patient Reported Outcome (PRO). Nonresponder imputation (NRI) was used. PBO patients who did not achieve PRO clinical response at W12 were switched to MIRI and analyzed as NRs for all W52 efficacy outcomes (PBO/PBO W12 Observation Carried Forward method) (Figure 1). In the USPI, PBO patients switched to MIRI at W12 were analyzed as PBO patients with their observed W52 results used for all W52 efficacy endpoints despite having received MIRI for 40 weeks (PBO/PBO + PBO/MIRI Observed method). To assess the impact of the two methods on MIRI effect size, we examined efficacy in the USPI population using both methods.
Results: For the endpoints included, W52 PBO rates were 5-17% greater for the observed method compared with the observation carried forward method, thereby changing MIRI effect size by the same amount (Figure 2). Across the four W52 endpoints, a range of 48-69% of the W52 PBO response rate for the observed method was driven by patients with 40 weeks treatment on MIRI.
Discussion: PBO rate differences between the MIRI USPI and published VIVID-1 analyses are due to different methodologic approaches to calculating treatment response. Published greater differences in the treatment effect between MIRI and PBO over 52 weeks included MIRI-treated patients but as NRs (40 weeks of MIRI treatment) in the PBO response rate, whereas these patients were included with observed data in the method used for the USPI.
References:
1. Ferrante et al., The Lancet 2024; 404:2423-36.
2. OMVOH (Mirikizumab-mrkz) [US PI]. Indianapolis, IN, USA: Eli Lilly and Company, 2025.

Figure: Figure 1. Methods of handling placebo in the VIVID-1 study manuscript and the USPI: Graphical comparison.1,2.
*NR result counted for MIRI result both in MS and USPI. Note: For the USPI, the PAS population inclusion criteria were a baseline Crohn’s Disease Activity Index (CDAI) total score ≥220 (1 clinical site was excluded); the SES-CD criteria were unchanged. This resulted in 31 fewer PBO and 68 fewer MIRI patients. Abbreviations: MIRI=Mirikizumab-mrkz; MS=Manuscript; NR=Nonresponders; PBO=Placebo; R=Responders; USPI=United States Package Insert; W=Week. 1. Ferrante et al., The Lancet 2024; 404:2423-36. 2. OMVOH (Mirikizumab-mrkz) [USPI]. Indianapolis, IN, USA: Eli Lilly and Company, 2025.

Figure: Figure 2. Efficacy at week 52 by placebo-handling method (VIVID-1 USPI population; NRI): Impact on mirikizumab effect size.
aThis includes all 168 patients randomized to PBO at baseline. Of those, 67 (40%) patients who did not achieve clinical response by PRO at W12 were switched to treatment with MIRI (designated as PBO/MIRI) and their efficacy data are included here with the remaining patients randomized to PBO who did not receive MIRI (designated as PBO). bFollowing MIRI 900 mg as an IV infusion at Week 0, Week 4, and Week 8, patients received MIRI 300 mg as a SC injection at W12 and every 4 weeks thereafter for up to an additional 40 weeks. CDAI=Crohn's Disease Activity Index; CS=Corticosteroid; IV=Intravenous; MIRI=Mirikizumab-mrkz; N=Number of Patients in the Analysis Population; n=Number of Patients in the Specified Category; NRI=Nonresponder Imputation; PBO=Placebo; PRO=Patient-Reported Outcome; SC=Subcutaneous; USPI=United States Package Insert; W=Week. 1. OMVOH (Mirikizumab-mrkz) [USPI]. Indianapolis, IN, USA: Eli Lilly and Company, 2025. 2. Ferrante et al., The Lancet 2024; 404:2423-36.
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Miguel Regueiro: AbbVie – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. BMS – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim Pharmaceuticals Inc. – Advisory Committee/Board Member, Consultant. Celegene – Advisory Committee/Board Member, Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Prometheus – Advisory Committee/Board Member, Consultant. Salix – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. UCB – Advisory Committee/Board Member, Consultant.
David Clemow: Eli Lilly and Company – Employee, Stock Options.
Deborah Fisher: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Richard Moses: Eli Lilly and Company – Employee, Stock Options.
Gary Lichtenstein: Abbvie – Consultant. American College of Gastroenterology – honorarium as associate editor of American Journal of Gastroenterology. Celgene – Consultant, Grant/Research Support. CellCeutrix – Consultant. Clinical Advances in Gastroenterology – Honorarium - Associate Editor. Eli Lilly – Consultant, DSMB. Ferring – Consultant. Gastroenterology and Hepatology [Gastro-Hep Communications] – Honorarium - Editor. Gilead – Consultant. Johnson & Johnson – Consultant. Luitpold/American Regent – Consultant, Honorarium - CME programs. McMahon Publishing – Honorarium - Author. Merck – Consultant, Honorarium - CME programs. Orthobiotech – Consultant, Grant/Research Support. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Romark – Consultant, Honorarium - CME programs. Salix/Valeant – Consultant, Grant/Research Support. Shire – Consultant, Grant/Research Support. SLACK – Book royalty. Springer – Honorarium - Editor. Takeda – Consultant, Funding to institution [IBD fellow education]. UCB – Consultant, Grant/Research Support. Up-To-Date – Honorarium - Author. Vindico – CME. Virgo – Consultant, Stock Options.
Bruce E. Sands, MD, MS, FACG1, Jessica R.. Allegretti, MD, MPH2, Miguel Regueiro, MD3, David B.. Clemow, PhD4, Deborah A.. Fisher, MD5, Guanglei Yu, PhD5, Richard E.. Moses, DO, JD4, Gary R.. Lichtenstein, MD, FACG6. P3252 - Mirikizumab Crohn's Disease VIVID-1 Study Placebo Response Differences Between the United States Labeling and Primary Manuscript Due to Methodological Differences in Handling Participants Randomized to Placebo, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
