Monday Poster Session
Category: IBD
P3249 - High Prevalence of Advanced Therapy Use in Patients With Crohn’s Disease in a Large Community and Academic Cohort: A Preliminary Analysis From IBD Qorus
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Sapana Gupta, MD
Brown University / Rhode Island Hospital
Providence, RI
Presenting Author(s)
Sapana Gupta, MD1, Adam A. Saleh, MD2, David Fudman, MD2, Ridhima Oberai, BS3, Corey A. Siegel, MD, MS4, Gil Y. Melmed, MD, MS, FACG5, Siddharth Singh, MD, MS6, Samir A. Shah, MD, FACG7
1Brown University / Rhode Island Hospital, Providence, RI; 2University of Texas Southwestern Medical Center, Dallas, TX; 3Crohn's and Colitis Foundation, New York, NY; 4Section of Gastroenterology and Hepatology, Dartmouth-Hitchcock Medical Center, Lebanon, NH; 5F. Widjaja Inflammatory Bowel Disease Institute, Cedars Sinai Medical Center, Los Angeles, CA; 6University of California San Diego, San Diego, CA; 7Gastroenterology Associates, Inc. powered by the GI alliance, Providence, RI
Introduction: Early effective therapy is well recognized to improve clinical outcomes in Crohn’s disease (CD), and underutilization of advanced therapies contributes to suboptimal outcomes. Most cohort studies describe advanced therapy (AT) use for only a minority of patients with CD. We hypothesized AT use may be more prevalent in IBD Qorus, a large multicenter learning health system.
Methods: Patient and provider survey responses from IBD Qorus were prospectively collected from November 2020 to May 2025 at 48 academic and community practices during routine clinical visits. We included patient visits with recorded current medication use and IBD classification of CD. We report descriptive statistics and a multivariable logistic regression with variable selection using the backward Wald method to identify factors associated with AT use. Differences in PRO3 between patients using versus not using advanced therapy were assessed with the Mann–Whitney U test.
Results: Among 16,515 included patient visits, which were divided evenly between community and academic sites, 79.4% (N= 13,105) reported advanced therapy use, including biologics in 76.4% (N=12,618), small molecules (i.e. JAK1 inhibitors) in 3.5% (N=579), and combination (two or more concurrent) AT in 1.2% (N=195) (Table 1).
Patients 71–80 years old (aOR = 0.52, 95% CI: 0.46–0.59), those taking steroids (aOR = 0.84, 95% CI: 0.71–0.99), and those who perceived a benefit from change in therapy (aOR = 0.64, 95% CI: 0.56–0.73) had significantly lower odds of advanced therapy use (Table 2). The absence of blood in stool was strongly associated with higher odds of advanced therapy use (aOR = 4.34, 95% CI: 4.09–4.66). The median PRO3 CD score was lower among those using AT versus those not using AT (7.00 vs. 9.00; p < 0.001).
Discussion: AT use for CD was reported in 79% of visits in this large cohort of both community and academic sites. To our knowledge, this high prevalence exceeds the use reported in other published cohorts. This finding may be explained by cohort members having a special interest in IBD or the effects of participation in the IBD Qorus collaborative. Further analyses should examine trends in advanced therapy utilization over time and across clinical settings, with a focus on patient outcomes to help inform and support the appropriate use of these therapies.
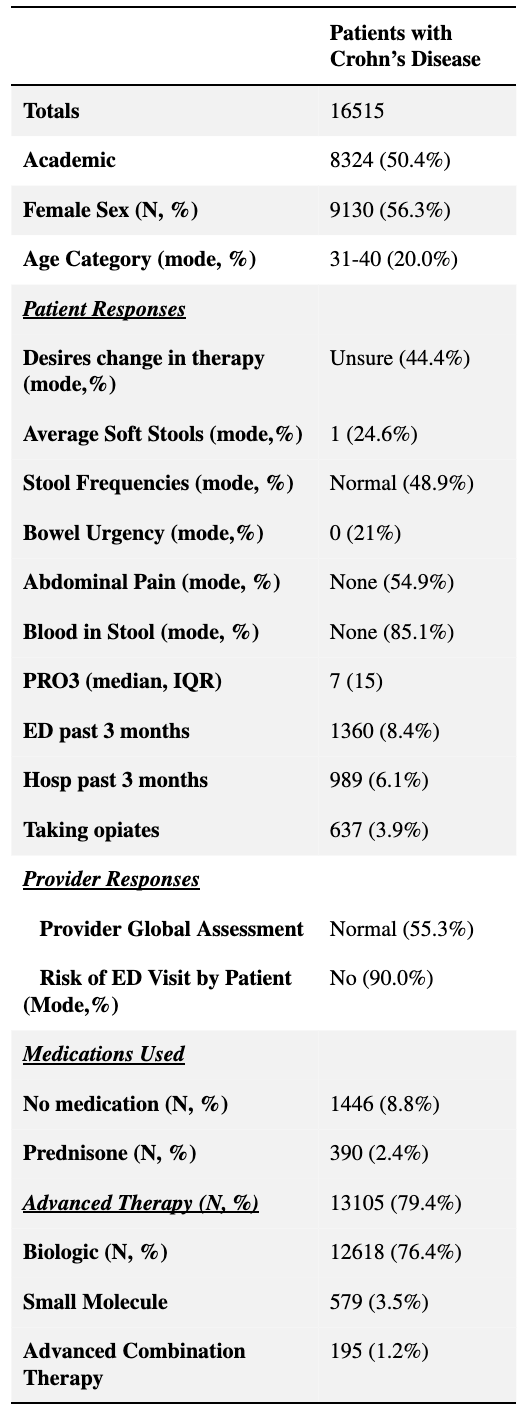
Figure: Table 1: Baseline characteristics of Qorus patients with Crohn’s disease
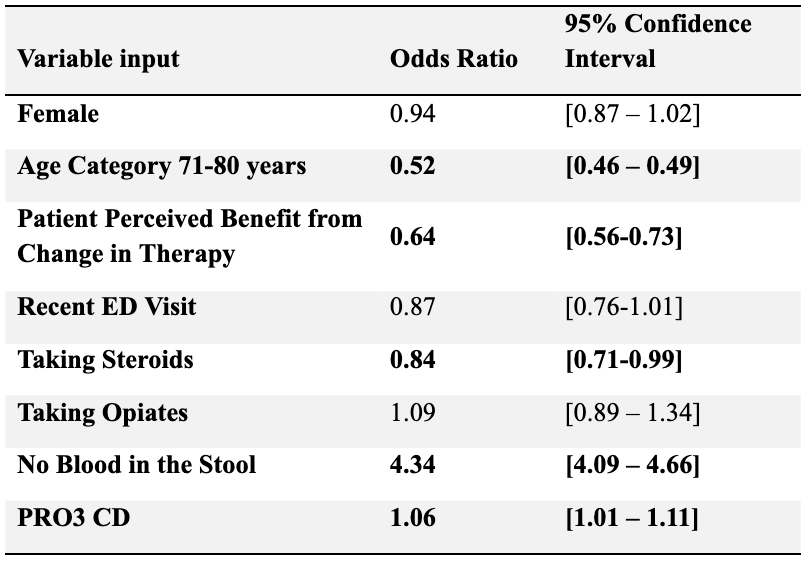
Figure: Table 2. Multivariate analysis for factors associated with advanced therapy use
Disclosures:
Sapana Gupta indicated no relevant financial relationships.
Adam Saleh indicated no relevant financial relationships.
David Fudman: Eli Lilly – Advisory Committee/Board Member. Fresenius Kabi – Consultant. Janssen – Advisory Committee/Board Member.
Ridhima Oberai indicated no relevant financial relationships.
Corey A. Siegel: AbbVie – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Boomerang – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Johnson and Johnson – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Lilly – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Napo Pharmaceuticals – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Path Healthcare – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Prometheus Labs – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Takeda – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Trellus Health – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau.
Gil Y. Melmed: AbbVie – Consultant. Arena – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Dieta – Consultant, Stock-privately held company. Ferring – Consultant. Fresenius Kalbi – Consultant. Genentech – Consultant. Gilead – Consultant. Iterative Scopes – Consultant. Janssen – Consultant. Lilly – Consultant. OptionCare – Personal Fees. Oshi Health – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus Labs – Consultant. Samsung Bioepis – Consultant. Takeda – Consultant. Techlab – Consultant. Treating inflammatory bowel disease using ultraviolet light – Intellectual Property/Patents. Treating inflammatory bowel disease with antifungal therapy – Intellectual Property/Patents. Verantos – Personal Fees. Viatris – Consultant.
Siddharth Singh indicated no relevant financial relationships.
Samir Shah: pfizer – Advisory Committee/Board Member. Roche Information Systems – Consultant.
Sapana Gupta, MD1, Adam A. Saleh, MD2, David Fudman, MD2, Ridhima Oberai, BS3, Corey A. Siegel, MD, MS4, Gil Y. Melmed, MD, MS, FACG5, Siddharth Singh, MD, MS6, Samir A. Shah, MD, FACG7. P3249 - High Prevalence of Advanced Therapy Use in Patients With Crohn’s Disease in a Large Community and Academic Cohort: A Preliminary Analysis From IBD Qorus, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Brown University / Rhode Island Hospital, Providence, RI; 2University of Texas Southwestern Medical Center, Dallas, TX; 3Crohn's and Colitis Foundation, New York, NY; 4Section of Gastroenterology and Hepatology, Dartmouth-Hitchcock Medical Center, Lebanon, NH; 5F. Widjaja Inflammatory Bowel Disease Institute, Cedars Sinai Medical Center, Los Angeles, CA; 6University of California San Diego, San Diego, CA; 7Gastroenterology Associates, Inc. powered by the GI alliance, Providence, RI
Introduction: Early effective therapy is well recognized to improve clinical outcomes in Crohn’s disease (CD), and underutilization of advanced therapies contributes to suboptimal outcomes. Most cohort studies describe advanced therapy (AT) use for only a minority of patients with CD. We hypothesized AT use may be more prevalent in IBD Qorus, a large multicenter learning health system.
Methods: Patient and provider survey responses from IBD Qorus were prospectively collected from November 2020 to May 2025 at 48 academic and community practices during routine clinical visits. We included patient visits with recorded current medication use and IBD classification of CD. We report descriptive statistics and a multivariable logistic regression with variable selection using the backward Wald method to identify factors associated with AT use. Differences in PRO3 between patients using versus not using advanced therapy were assessed with the Mann–Whitney U test.
Results: Among 16,515 included patient visits, which were divided evenly between community and academic sites, 79.4% (N= 13,105) reported advanced therapy use, including biologics in 76.4% (N=12,618), small molecules (i.e. JAK1 inhibitors) in 3.5% (N=579), and combination (two or more concurrent) AT in 1.2% (N=195) (Table 1).
Patients 71–80 years old (aOR = 0.52, 95% CI: 0.46–0.59), those taking steroids (aOR = 0.84, 95% CI: 0.71–0.99), and those who perceived a benefit from change in therapy (aOR = 0.64, 95% CI: 0.56–0.73) had significantly lower odds of advanced therapy use (Table 2). The absence of blood in stool was strongly associated with higher odds of advanced therapy use (aOR = 4.34, 95% CI: 4.09–4.66). The median PRO3 CD score was lower among those using AT versus those not using AT (7.00 vs. 9.00; p < 0.001).
Discussion: AT use for CD was reported in 79% of visits in this large cohort of both community and academic sites. To our knowledge, this high prevalence exceeds the use reported in other published cohorts. This finding may be explained by cohort members having a special interest in IBD or the effects of participation in the IBD Qorus collaborative. Further analyses should examine trends in advanced therapy utilization over time and across clinical settings, with a focus on patient outcomes to help inform and support the appropriate use of these therapies.

Figure: Table 1: Baseline characteristics of Qorus patients with Crohn’s disease

Figure: Table 2. Multivariate analysis for factors associated with advanced therapy use
Disclosures:
Sapana Gupta indicated no relevant financial relationships.
Adam Saleh indicated no relevant financial relationships.
David Fudman: Eli Lilly – Advisory Committee/Board Member. Fresenius Kabi – Consultant. Janssen – Advisory Committee/Board Member.
Ridhima Oberai indicated no relevant financial relationships.
Corey A. Siegel: AbbVie – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Boomerang – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Johnson and Johnson – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Lilly – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Napo Pharmaceuticals – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Path Healthcare – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Pfizer – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Prometheus Labs – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Sanofi – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Takeda – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau. Trellus Health – Advisor or Review Panel Member, Consultant, Grant/Research Support, Speakers Bureau.
Gil Y. Melmed: AbbVie – Consultant. Arena – Consultant. Boehringer Ingelheim – Consultant. Bristol Myers Squibb – Consultant. Dieta – Consultant, Stock-privately held company. Ferring – Consultant. Fresenius Kalbi – Consultant. Genentech – Consultant. Gilead – Consultant. Iterative Scopes – Consultant. Janssen – Consultant. Lilly – Consultant. OptionCare – Personal Fees. Oshi Health – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus Labs – Consultant. Samsung Bioepis – Consultant. Takeda – Consultant. Techlab – Consultant. Treating inflammatory bowel disease using ultraviolet light – Intellectual Property/Patents. Treating inflammatory bowel disease with antifungal therapy – Intellectual Property/Patents. Verantos – Personal Fees. Viatris – Consultant.
Siddharth Singh indicated no relevant financial relationships.
Samir Shah: pfizer – Advisory Committee/Board Member. Roche Information Systems – Consultant.
Sapana Gupta, MD1, Adam A. Saleh, MD2, David Fudman, MD2, Ridhima Oberai, BS3, Corey A. Siegel, MD, MS4, Gil Y. Melmed, MD, MS, FACG5, Siddharth Singh, MD, MS6, Samir A. Shah, MD, FACG7. P3249 - High Prevalence of Advanced Therapy Use in Patients With Crohn’s Disease in a Large Community and Academic Cohort: A Preliminary Analysis From IBD Qorus, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

