Monday Poster Session
Category: IBD
P3242 - Vedolizumab Demonstrated Significant Improvements in Symptomatic Relief and Quality of Life in Patients With Crohn’s Disease: Results from VALUE, a Prospective, Multicenter, Observational Study
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- XQ
Xiaoyu Qian, MSc
Takeda Pharmaceutical Company, Shanghai, China
Shanghai, Shanghai, China
Presenting Author(s)
Changqing Zheng, MD1, Lan Zhong, MD2, Yingde Wang, MD3, Xiaoyu Qian, MSc4, Fang Zhou, MSc4,Minhu Chen, MD, PhD5
1China Medical University Affiliated Shengjing Hospital, Shenyang, China, Shenyang, Liaoning, China; 2Shanghai East Hospital, Tongji University School of Medicine, Shanghai, China, Shanghai, Shanghai, China; 3The First Affiliated Hospital of Dalian Medical University, Dalian, China, Dalian, Liaoning, China; 4Takeda Pharmaceutical Company, Shanghai, China, Shanghai, Shanghai, China; 5The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China, Guangzhou, Guangdong, China
Introduction: Vedolizumab, a gut-selective monoclonal antibody targeting α4β7 integrin, is approved in China for the treatment of moderate-to-severe ulcerative colitis and Crohn’s disease (CD) in adult patients. Using patient-reported outcomes (PROs), we assessed the effectiveness of vedolizumab in improving symptomatic relief and quality of life (QoL) in Chinese patients with CD from the VALUE study (NCT04872491).
Methods: VALUE is a prospective, multicenter, single-arm, observational study. Enrolled patients with CD aged ≥18 years were treated with 300 mg vedolizumab by intravenous infusion at Weeks 0, 2, and 6, and every 8 weeks until 54 weeks. In this final analysis, the abdominal pain subscore (APS) and loose stool frequency subscore (LSFS) were assessed at baseline and on Days 2–14. Harvey-Bradshaw Index (HBI) score was assessed at baseline, Week 14, 30 and 54. Inflammatory Bowel Disease Questionnaire (IBDQ), EuroQoL 5‑Dimension 5-Level (EQ-5D-5L), and EuroQoL-Visual Analog Scale (EQ-VAS) scores were analyzed at baseline, Week 14, and Week 54. All analyses were descriptive.
Results: Of the 91 patients with CD enrolled, 89 (97.8%) were included in the effectiveness analysis set. The mean (standard deviation [SD]) age at baseline was 35.3 (11.86) years and 70.3% were male. The majority (81.3%) of patients were nonsmokers. Of the total enrolled patients with CD, 34 (37.4%) had prior exposure to biologics. At baseline, the mean (SD) HBI score was 5.6 (3.35); the mean (SD) APS and LSFS were 0.7 (0.88) and 1.6 (1.62), respectively. The percentage of patients with APS≤1 and LSFS≤3 increased from 75.0% at baseline to 78.1% by Day 2 and 85.5% by Day 14. The mean HBI scores from baseline to Week 54 are presented in Figure 1. Vedolizumab demonstrated improved QoL in patients with CD from baseline at Week 14, which was sustained up to Week 54 (Table 1). At Weeks 14 and 54, the mean change (95% confidence interval) from baseline in the IBDQ total score was 19.5 (11.26, 27.74) and 26.4 (15.93, 36.89); in the EQ-5D-5L total score, it was 0.04 (0.01, 0.07) and 0.04 (0.00, 0.09); and in the EQ-VAS score, it was 6.0 (2.16, 9.81) and 6.8 (1.64, 12.03), respectively.
Discussion: Vedolizumab treatment demonstrated improvements in PROs for symptom relief and QoL in Chinese patients with CD in real-world settings.
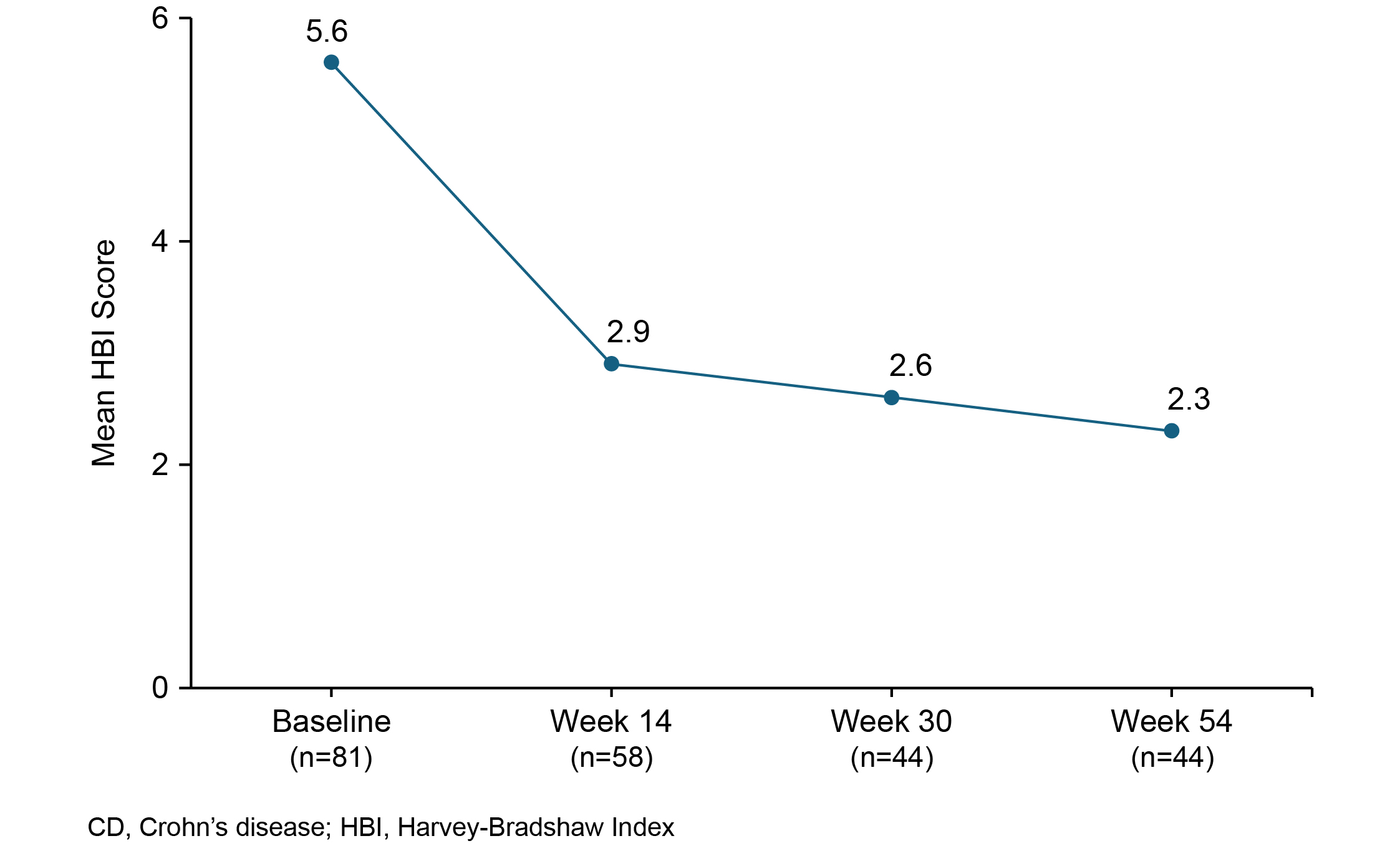
Figure: Figure 1: Mean HBI score — at baseline, Week 14, Week 30 and Week 54 in patients with CD

Figure: Table 1. IBDQ, EQ-5D-5L, and EQ-VAS scores at baseline, Week 14, and Week 54
Disclosures:
Changqing Zheng: Takeda (China) International Trading Co. Ltd – Grant/Research Support.
Lan Zhong: Takeda (China) International Trading Co. Ltd – Grant/Research Support.
Yingde Wang: Takeda (China) International Trading Co. Ltd – Grant/Research Support.
Xiaoyu Qian: Takeda (China) International Trading Co. Ltd – Employee, Stock Options.
Fang Zhou: Takeda (China) International Trading Co. Ltd – Employee, Stock Options.
Minhu Chen: AstraZeneca China – Speaker honoraria. Eisai China – Speaker honoraria. Takeda (China) International Trading Co. Ltd – Grant/Research Support, Royalties. Xian Janssen – Speaker honoraria.
Changqing Zheng, MD1, Lan Zhong, MD2, Yingde Wang, MD3, Xiaoyu Qian, MSc4, Fang Zhou, MSc4,Minhu Chen, MD, PhD5. P3242 - Vedolizumab Demonstrated Significant Improvements in Symptomatic Relief and Quality of Life in Patients With Crohn’s Disease: Results from VALUE, a Prospective, Multicenter, Observational Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1China Medical University Affiliated Shengjing Hospital, Shenyang, China, Shenyang, Liaoning, China; 2Shanghai East Hospital, Tongji University School of Medicine, Shanghai, China, Shanghai, Shanghai, China; 3The First Affiliated Hospital of Dalian Medical University, Dalian, China, Dalian, Liaoning, China; 4Takeda Pharmaceutical Company, Shanghai, China, Shanghai, Shanghai, China; 5The First Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China, Guangzhou, Guangdong, China
Introduction: Vedolizumab, a gut-selective monoclonal antibody targeting α4β7 integrin, is approved in China for the treatment of moderate-to-severe ulcerative colitis and Crohn’s disease (CD) in adult patients. Using patient-reported outcomes (PROs), we assessed the effectiveness of vedolizumab in improving symptomatic relief and quality of life (QoL) in Chinese patients with CD from the VALUE study (NCT04872491).
Methods: VALUE is a prospective, multicenter, single-arm, observational study. Enrolled patients with CD aged ≥18 years were treated with 300 mg vedolizumab by intravenous infusion at Weeks 0, 2, and 6, and every 8 weeks until 54 weeks. In this final analysis, the abdominal pain subscore (APS) and loose stool frequency subscore (LSFS) were assessed at baseline and on Days 2–14. Harvey-Bradshaw Index (HBI) score was assessed at baseline, Week 14, 30 and 54. Inflammatory Bowel Disease Questionnaire (IBDQ), EuroQoL 5‑Dimension 5-Level (EQ-5D-5L), and EuroQoL-Visual Analog Scale (EQ-VAS) scores were analyzed at baseline, Week 14, and Week 54. All analyses were descriptive.
Results: Of the 91 patients with CD enrolled, 89 (97.8%) were included in the effectiveness analysis set. The mean (standard deviation [SD]) age at baseline was 35.3 (11.86) years and 70.3% were male. The majority (81.3%) of patients were nonsmokers. Of the total enrolled patients with CD, 34 (37.4%) had prior exposure to biologics. At baseline, the mean (SD) HBI score was 5.6 (3.35); the mean (SD) APS and LSFS were 0.7 (0.88) and 1.6 (1.62), respectively. The percentage of patients with APS≤1 and LSFS≤3 increased from 75.0% at baseline to 78.1% by Day 2 and 85.5% by Day 14. The mean HBI scores from baseline to Week 54 are presented in Figure 1. Vedolizumab demonstrated improved QoL in patients with CD from baseline at Week 14, which was sustained up to Week 54 (Table 1). At Weeks 14 and 54, the mean change (95% confidence interval) from baseline in the IBDQ total score was 19.5 (11.26, 27.74) and 26.4 (15.93, 36.89); in the EQ-5D-5L total score, it was 0.04 (0.01, 0.07) and 0.04 (0.00, 0.09); and in the EQ-VAS score, it was 6.0 (2.16, 9.81) and 6.8 (1.64, 12.03), respectively.
Discussion: Vedolizumab treatment demonstrated improvements in PROs for symptom relief and QoL in Chinese patients with CD in real-world settings.

Figure: Figure 1: Mean HBI score — at baseline, Week 14, Week 30 and Week 54 in patients with CD

Figure: Table 1. IBDQ, EQ-5D-5L, and EQ-VAS scores at baseline, Week 14, and Week 54
Disclosures:
Changqing Zheng: Takeda (China) International Trading Co. Ltd – Grant/Research Support.
Lan Zhong: Takeda (China) International Trading Co. Ltd – Grant/Research Support.
Yingde Wang: Takeda (China) International Trading Co. Ltd – Grant/Research Support.
Xiaoyu Qian: Takeda (China) International Trading Co. Ltd – Employee, Stock Options.
Fang Zhou: Takeda (China) International Trading Co. Ltd – Employee, Stock Options.
Minhu Chen: AstraZeneca China – Speaker honoraria. Eisai China – Speaker honoraria. Takeda (China) International Trading Co. Ltd – Grant/Research Support, Royalties. Xian Janssen – Speaker honoraria.
Changqing Zheng, MD1, Lan Zhong, MD2, Yingde Wang, MD3, Xiaoyu Qian, MSc4, Fang Zhou, MSc4,Minhu Chen, MD, PhD5. P3242 - Vedolizumab Demonstrated Significant Improvements in Symptomatic Relief and Quality of Life in Patients With Crohn’s Disease: Results from VALUE, a Prospective, Multicenter, Observational Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
