Monday Poster Session
Category: IBD
P3195 - Treat-to-Target Gaps in Crohn’s Disease: Insights From Real-World Practice
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Bruce E. Sands, MD, MS, FACG
Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
New York, NY
Presenting Author(s)
Bruce E. Sands, MD, MS, FACG1, Yasmmyn D. Salinas, PhD, MPH2, Teresa Bufford, PhD2, Julie M. Crawford, MD2, Derek R. Gazis, MS2, Kathleen Hurwitz, ScD2, Ebuwa Igho-Osagie, MBBS, DPH, MBA3, Gui Liu, PhD, MPH4, Jeremy Shao, MD, PhD5, Millie D. Long, MD, FACG6
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Target RWE, Durham, NC; 3Merck, Rahway, NJ; 4Merck Sharp & Dohme LLC, Philadelphia, PA; 5Merck & Co, South San Francisco, CA; 6Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC
Introduction: Treat-to-target strategies in Crohn’s disease (CD) emphasize objective monitoring to assess therapeutic response and guide early therapeutic escalation. However, data on real-world application of treat-to-target recommendations remains limited. We evaluated post-treatment monitoring, remission, and treatment patterns during the first year after initiation of advanced therapy (AT) for CD.
Methods: We analyzed data from TARGET-IBD, a longitudinal U.S. registry of patients with clinician-diagnosed IBD. Adult patients initiating a new AT (anti-IL-12/23, anti-integrin, anti-TNF, JAK inhibitors, S1P modulators) between October 2014 and September 2021 were included for follow-up. Objective reassessment was defined as endoscopy or fecal calprotectin (FC) testing within 12 months. Remission was evaluated at 2–4 and 10–14 months post-initiation (mimicking induction and maintenance windows in clinical trials). Outcomes included endoscopic remission (SES-CD 0–4, or 0–3 for ileal-only disease, or narrative indication of inactive disease) and corticosteroid-free remission (no systemic corticosteroid use in the 14 days before endoscopy), overall and by line of advanced therapy (first-line [1L] AT vs. second-line or later [2L+] AT). Treatment persistence and corticosteroid use were also summarized.
Results: Among 1189 CD patients, 38% underwent objective reassessment within 12 months; FC testing was rare (12%). At AT initiation, 39% had inflammatory CD, 35% fistulizing, and 27% stricturing disease. Ileocolonic disease was most common (56%), followed by isolated ileal (27%) and colonic (17%). At 2–4 months, only 55 patients (5%) underwent endoscopy: 31% achieved endoscopic remission and 26% corticosteroid-free remission (Figure 1). At 10–14 months, 156 patients (13%) underwent endoscopy: 45% achieved endoscopic remission and 39% corticosteroid-free remission. At this time window, remission proportions were higher for patients on 1L AT compared to 2L+ AT. Among patients with endoscopy at 10-14 months, 46% required at least one corticosteroid prescription after initiating AT. At 10-14 months, 33% had switched or discontinued their initial AT.
Discussion: In this national registry cohort of CD patients initiating a new AT, objective monitoring was infrequent, and corticosteroid use was high over the first year. Remission rates were modest, and many patients required treatment changes. These findings highlight real-world gaps in treat-to-target implementation and early care optimization.
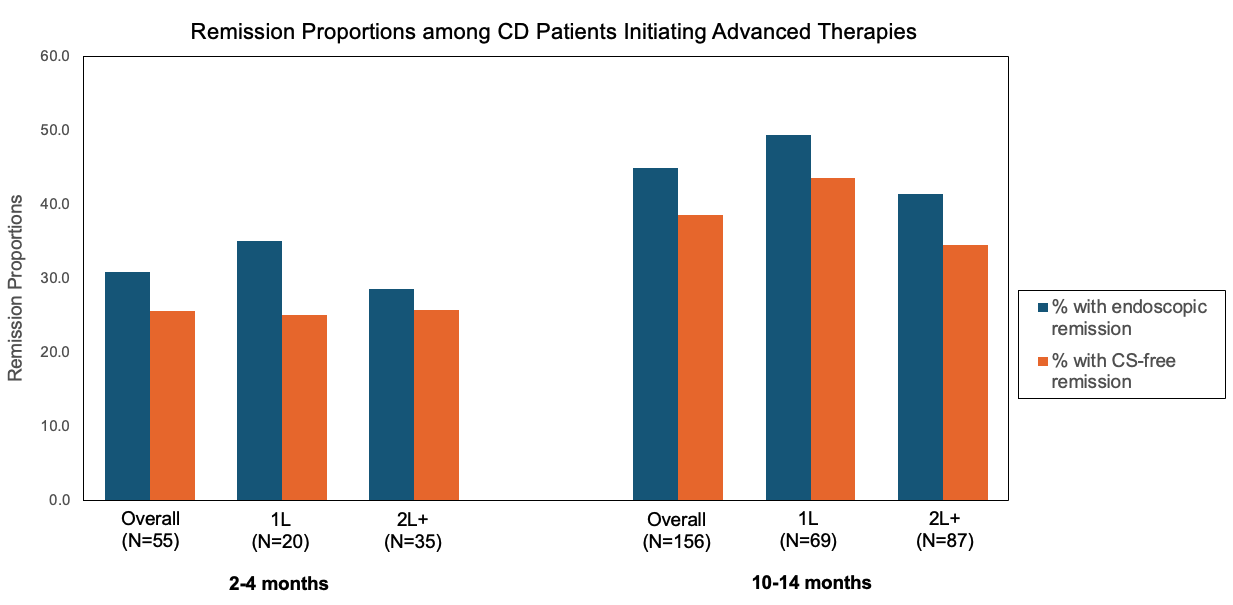
Figure: Figure 1. Remission proportions among Crohn’s disease (CD) patients initiating advanced therapies. Bar graph shows the percentage of patients achieving endoscopic remission (blue) and corticosteroid (CS)-free remission (orange) at 2–4 months and 10–14 months post-treatment initiation, stratified by line of advanced therapy (1L: first-line advanced therapy ; 2L+: second-line or later advanced therapy).
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Yasmmyn Salinas: Target RWE – Employee, Stock Options.
Teresa Bufford: Target RWE – Employee, Stock Options.
Julie Crawford: Target RWE – Employee, Stock Options.
Derek Gazis: Target RWE – Employee, Stock Options.
Kathleen Hurwitz: Target RWE – Employee, Stock Options.
Ebuwa Igho-Osagie: Merck & Co., Inc., Rahway, NJ, USA – Employee.
Gui Liu: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc – Employee.
Jeremy Shao: Merck Sharp & Dohme LLC – Employee, Stock Options.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Bruce E. Sands, MD, MS, FACG1, Yasmmyn D. Salinas, PhD, MPH2, Teresa Bufford, PhD2, Julie M. Crawford, MD2, Derek R. Gazis, MS2, Kathleen Hurwitz, ScD2, Ebuwa Igho-Osagie, MBBS, DPH, MBA3, Gui Liu, PhD, MPH4, Jeremy Shao, MD, PhD5, Millie D. Long, MD, FACG6. P3195 - Treat-to-Target Gaps in Crohn’s Disease: Insights From Real-World Practice, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Target RWE, Durham, NC; 3Merck, Rahway, NJ; 4Merck Sharp & Dohme LLC, Philadelphia, PA; 5Merck & Co, South San Francisco, CA; 6Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC
Introduction: Treat-to-target strategies in Crohn’s disease (CD) emphasize objective monitoring to assess therapeutic response and guide early therapeutic escalation. However, data on real-world application of treat-to-target recommendations remains limited. We evaluated post-treatment monitoring, remission, and treatment patterns during the first year after initiation of advanced therapy (AT) for CD.
Methods: We analyzed data from TARGET-IBD, a longitudinal U.S. registry of patients with clinician-diagnosed IBD. Adult patients initiating a new AT (anti-IL-12/23, anti-integrin, anti-TNF, JAK inhibitors, S1P modulators) between October 2014 and September 2021 were included for follow-up. Objective reassessment was defined as endoscopy or fecal calprotectin (FC) testing within 12 months. Remission was evaluated at 2–4 and 10–14 months post-initiation (mimicking induction and maintenance windows in clinical trials). Outcomes included endoscopic remission (SES-CD 0–4, or 0–3 for ileal-only disease, or narrative indication of inactive disease) and corticosteroid-free remission (no systemic corticosteroid use in the 14 days before endoscopy), overall and by line of advanced therapy (first-line [1L] AT vs. second-line or later [2L+] AT). Treatment persistence and corticosteroid use were also summarized.
Results: Among 1189 CD patients, 38% underwent objective reassessment within 12 months; FC testing was rare (12%). At AT initiation, 39% had inflammatory CD, 35% fistulizing, and 27% stricturing disease. Ileocolonic disease was most common (56%), followed by isolated ileal (27%) and colonic (17%). At 2–4 months, only 55 patients (5%) underwent endoscopy: 31% achieved endoscopic remission and 26% corticosteroid-free remission (Figure 1). At 10–14 months, 156 patients (13%) underwent endoscopy: 45% achieved endoscopic remission and 39% corticosteroid-free remission. At this time window, remission proportions were higher for patients on 1L AT compared to 2L+ AT. Among patients with endoscopy at 10-14 months, 46% required at least one corticosteroid prescription after initiating AT. At 10-14 months, 33% had switched or discontinued their initial AT.
Discussion: In this national registry cohort of CD patients initiating a new AT, objective monitoring was infrequent, and corticosteroid use was high over the first year. Remission rates were modest, and many patients required treatment changes. These findings highlight real-world gaps in treat-to-target implementation and early care optimization.

Figure: Figure 1. Remission proportions among Crohn’s disease (CD) patients initiating advanced therapies. Bar graph shows the percentage of patients achieving endoscopic remission (blue) and corticosteroid (CS)-free remission (orange) at 2–4 months and 10–14 months post-treatment initiation, stratified by line of advanced therapy (1L: first-line advanced therapy ; 2L+: second-line or later advanced therapy).
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Yasmmyn Salinas: Target RWE – Employee, Stock Options.
Teresa Bufford: Target RWE – Employee, Stock Options.
Julie Crawford: Target RWE – Employee, Stock Options.
Derek Gazis: Target RWE – Employee, Stock Options.
Kathleen Hurwitz: Target RWE – Employee, Stock Options.
Ebuwa Igho-Osagie: Merck & Co., Inc., Rahway, NJ, USA – Employee.
Gui Liu: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc – Employee.
Jeremy Shao: Merck Sharp & Dohme LLC – Employee, Stock Options.
Millie Long: AbbVie – Consultant. Bristol Myers Squibb – Consultant. Celltrion – Consultant, Grant/Research Support. Intercept – Consultant. Johnson & Johnson – Consultant. Lilly – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant, Grant/Research Support. Prometheus – Consultant. Roivant – Consultant. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant, Grant/Research Support. Target RWE – Consultant.
Bruce E. Sands, MD, MS, FACG1, Yasmmyn D. Salinas, PhD, MPH2, Teresa Bufford, PhD2, Julie M. Crawford, MD2, Derek R. Gazis, MS2, Kathleen Hurwitz, ScD2, Ebuwa Igho-Osagie, MBBS, DPH, MBA3, Gui Liu, PhD, MPH4, Jeremy Shao, MD, PhD5, Millie D. Long, MD, FACG6. P3195 - Treat-to-Target Gaps in Crohn’s Disease: Insights From Real-World Practice, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
