Monday Poster Session
Category: IBD
P3194 - Efficacy and Safety of Tulisokibart Maintenance Treatment in Tulisokibart Induction, Re-induction, and Delayed Induction Responders: Open-Label Extension of the ARTEMIS-UC Trial in Patients With Ulcerative Colitis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Bruce E. Sands, MD, MS, FACG
Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA
New York, NY
Presenting Author(s)
Bruce E. Sands, MD, MS, FACG1, Sami Hoque, MD, PhD2, Brian G. Feagan, MD3, Mark Yen, 4, Bin Dong, 4, Wen Zhou, 4, Silvio Danese, MD, PhD5
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Department of Gastroenterology, Barts Health NHS Trust, London, England, United Kingdom; 3Division of Gastroenterology, Department of Medicine, University of Western Ontario and Alimentiv, London, ON, Canada; 4Merck & Co., Inc., Rahway, NJ; 5Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy
Introduction: In the phase 2 ARTEMIS-UC study, a significantly higher proportion of participants on tulisokibart compared to placebo achieved clinical remission after 12 weeks of induction treatment in patients with moderately to severely active ulcerative colitis (UC).1 This post-hoc analysis evaluates efficacy and safety in initial non-responders to 12-week induction dosing who achieved response upon re-induction and were given maintenance dosing in an open-label extension (OLE).
Methods: Participants (≥18 years) were randomized to intravenous tulisokibart 12-week induction (1000 mg on day 1 and 500 mg at weeks 2, 6, and 10) or placebo and tested for anti-TL1A response likelihood. This analysis was done in Cohort 1 (positive and negative participants). Participants were classified as induction responders (reduction of ≥2 points and ≥30% in modified Mayo score from baseline, and a reduction ≥1 in rectal bleeding subscore or absolute rectal bleeding subscore ≤1 at week 12) or non-responders. Induction non-responders underwent a 12-week re-induction (or delayed induction for initial placebo participants) with open-label tulisokibart induction dosing. Cohort 1 induction, re-induction, and delayed induction responders were randomized to OLE maintenance dosing (tulisokibart 100 mg or 250 mg every 4 weeks) up to Week 50.
Results: In Cohort 1, 47 tulisokibart induction responders, 13 re-induction responders, and 29 delayed induction responders were randomized to OLE maintenance treatment with tulisokibart. Maintenance of treatment effect was generally similar with a trend for higher efficacy with tulisokibart 250 mg vs 100 mg, observed across the 3 groups (Table). The safety profile of tulisokibart maintenance treatment was similar across the 3 groups, with no new safety signals being observed.
Discussion: Tulisokibart was effective as a maintenance treatment in patients with moderately to severely active UC. The effect was generally similar across Cohort 1 tulisokibart induction, re-induction, and delayed induction responders. Tulisokibart maintenance treatment was well tolerated across the 3 groups. Taken altogether, a second induction regimen for tulisokibart non-responders may result in a long-term efficacy benefit without an increased safety risk. Larger trials are needed to confirm these findings.
References:
1. Sands BE et al. N Engl J Med. 2024; 391:1119─1129.
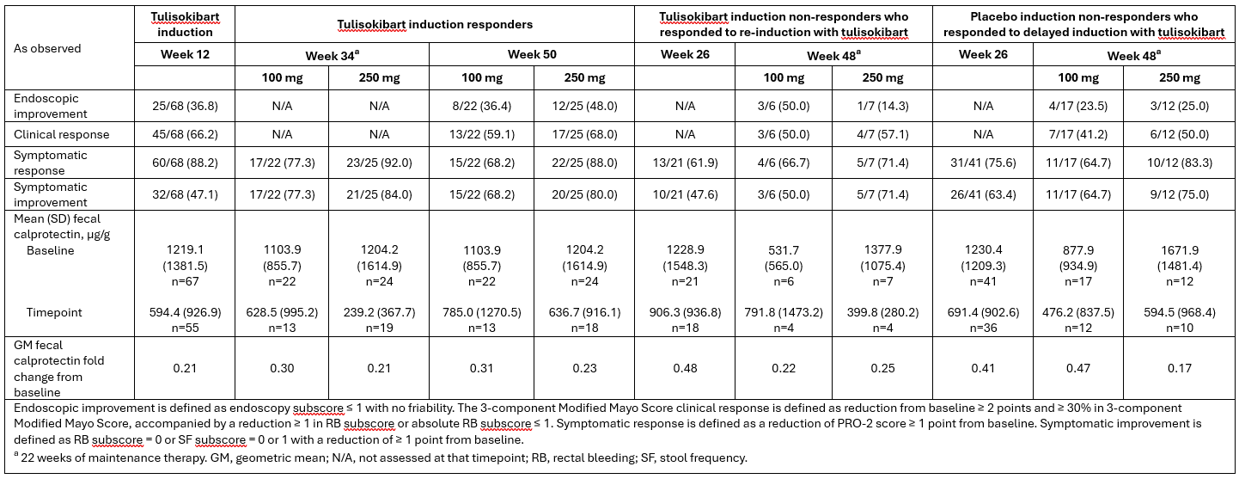
Figure: Table: Efficacy and fecal calprotectin
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Sami Hoque: MSD – Grant/Research Support.
Brian G. Feagan: Abbvie – Advisory Committee/Board Member, Consultant, honoraria. Abivax – Consultant. Adiso – Consultant. AgomAb Therapeutics – Consultant. Allianthera – Consultant. Amgen – Consultant. AnaptysBio – Advisory Committee/Board Member, Consultant. Arena Pharma – Consultant. Atomwise – Consultant. Avoro Capital Advisors – Consultant. BioJamp – Consultant. Biora Therapeutics – Consultant. Blackbird Laboratories – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Boxer Capital – Consultant. Celgene/BMS – Advisory Committee/Board Member, Consultant. Connect BioPharma – Consultant, Stock-publicly held company(excluding mutual/index funds). Cytoki – Consultant. Disc Medicine – Consultant. Duality – Consultant. EcoR1Capital – Advisory Committee/Board Member, Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. EnGene – Stock-publicly held company(excluding mutual/index funds). Equillium – Consultant. Ermium – Consultant. Evida – Stock-privately held company. First Wave – Consultant. Forbion – Consultant. Galapagos – Consultant. Galen Atlantica – Consultant. Genentech/Roche – Advisory Committee/Board Member, Consultant. Gilead – Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Gossamer Pharma – Consultant. Hinge Bio – Consultant. Imhotex – Consultant. Immunic Therapeutics – Consultant. Index Pharma – Consultant. Intercept – Consultant. JAKAcademy – Consultant. Janssen – Advisory Committee/Board Member, Consultant, honoraria; expert testimony. Japan Tobacco Inc. – Consultant. Kaleido Biosciences – Consultant. Klick Health – Consultant. Landos Biopharma – Consultant. Lenczner Slaght – Consultant. LifeSci Capital – Consultant. Lument AB – Consultant. Mage Biologics – Consultant. Merck – Advisory Committee/Board Member. Mestag – Consultant. Millennium – Consultant. MiroBio – Advisory Committee/Board Member, Consultant. Monte Rosa Therapeutics – Consultant. Morgan Lewis – Consultant. Morphic Therapeutics – Advisory Committee/Board Member, Consultant. Mylan – Consultant. Nexera – Consultant. Nexys Therapeutics – Consultant. Nimbus Therapeutics – Consultant. Odyssey – Consultant. OM Pharma – Consultant. OrbiMed – Consultant. Origo BioPharma – Advisory Committee/Board Member, Consultant. Orphagen – Consultant. Pandion Therapeutics – Consultant. Pendopharm – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, honoraria. Progenity – Consultant. Prometheus Therapeutics and Diagnostics (Merck & Co., Inc., Rahway, NJ, USA) – Consultant. Protagonist – Consultant. PTM Therapeutics – Consultant. Q32 Bio – Consultant. Rebiotix – Consultant. REDX Pharma – Advisory Committee/Board Member, Consultant. Roche – Consultant. Roivant/Televant – Consultant. Sandoz – Consultant. Sanofi – Advisory Committee/Board Member, Consultant. Seres Therapeutics – Consultant. Silverback Therapeutics – Consultant. Sobi – Consultant. Spyre Therapeutics – Consultant. Surrozen Inc. – Consultant. Synedgen – Consultant. Takeda – Advisory Committee/Board Member, Consultant, honoraria. Teva – Advisory Committee/Board Member, Consultant. Thelium – Consultant. Tigenix – Consultant. Tillotts – Consultant. Triastek – Consultant. Ventyx – Consultant.
Mark Yen: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. – Employee, Stock Options.
Bin Dong: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. – Employee, Stock Options.
Wen Zhou: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. – Employee, Stock Options.
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
Bruce E. Sands, MD, MS, FACG1, Sami Hoque, MD, PhD2, Brian G. Feagan, MD3, Mark Yen, 4, Bin Dong, 4, Wen Zhou, 4, Silvio Danese, MD, PhD5. P3194 - Efficacy and Safety of Tulisokibart Maintenance Treatment in Tulisokibart Induction, Re-induction, and Delayed Induction Responders: Open-Label Extension of the ARTEMIS-UC Trial in Patients With Ulcerative Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Department of Gastroenterology, Barts Health NHS Trust, London, England, United Kingdom; 3Division of Gastroenterology, Department of Medicine, University of Western Ontario and Alimentiv, London, ON, Canada; 4Merck & Co., Inc., Rahway, NJ; 5Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milan, Lombardia, Italy
Introduction: In the phase 2 ARTEMIS-UC study, a significantly higher proportion of participants on tulisokibart compared to placebo achieved clinical remission after 12 weeks of induction treatment in patients with moderately to severely active ulcerative colitis (UC).1 This post-hoc analysis evaluates efficacy and safety in initial non-responders to 12-week induction dosing who achieved response upon re-induction and were given maintenance dosing in an open-label extension (OLE).
Methods: Participants (≥18 years) were randomized to intravenous tulisokibart 12-week induction (1000 mg on day 1 and 500 mg at weeks 2, 6, and 10) or placebo and tested for anti-TL1A response likelihood. This analysis was done in Cohort 1 (positive and negative participants). Participants were classified as induction responders (reduction of ≥2 points and ≥30% in modified Mayo score from baseline, and a reduction ≥1 in rectal bleeding subscore or absolute rectal bleeding subscore ≤1 at week 12) or non-responders. Induction non-responders underwent a 12-week re-induction (or delayed induction for initial placebo participants) with open-label tulisokibart induction dosing. Cohort 1 induction, re-induction, and delayed induction responders were randomized to OLE maintenance dosing (tulisokibart 100 mg or 250 mg every 4 weeks) up to Week 50.
Results: In Cohort 1, 47 tulisokibart induction responders, 13 re-induction responders, and 29 delayed induction responders were randomized to OLE maintenance treatment with tulisokibart. Maintenance of treatment effect was generally similar with a trend for higher efficacy with tulisokibart 250 mg vs 100 mg, observed across the 3 groups (Table). The safety profile of tulisokibart maintenance treatment was similar across the 3 groups, with no new safety signals being observed.
Discussion: Tulisokibart was effective as a maintenance treatment in patients with moderately to severely active UC. The effect was generally similar across Cohort 1 tulisokibart induction, re-induction, and delayed induction responders. Tulisokibart maintenance treatment was well tolerated across the 3 groups. Taken altogether, a second induction regimen for tulisokibart non-responders may result in a long-term efficacy benefit without an increased safety risk. Larger trials are needed to confirm these findings.
References:
1. Sands BE et al. N Engl J Med. 2024; 391:1119─1129.

Figure: Table: Efficacy and fecal calprotectin
Disclosures:
Bruce Sands: AbbVie – Consultant. Abivax – Consultant, speaking fees. Adiso Therapeutics – Consultant. Agomab Therapeutics – Consultant. Alimentiv – Consultant. Amgen – Consultant. AnaptysBio – Consultant. Arena Pharmaceuticals – Consultant. Artugen Therapeutics – Consultant. Astra Zeneca – Consultant. Biolojic Design – Consultant. Biora Therapeutics – Consultant. Boehringer Ingelheim – Consultant. Boston Pharmaceuticals – Consultant. Bristol Myers Squibb – Consultant, Grant/Research Support, speaking fees. Calibr – Consultant. Celgene – Consultant. Celltrion – Consultant. ClostraBio – Consultant. Eli Lilly & Company – Consultant, speaking fees. Enthera – Consultant. Enveda Biosciences – Consultant. Equillium – Consultant. Evommune – Consultant. Ferring – Consultant. Fresenius Kabi – Consultant. Fzatat – Consultant. Galapagos – Consultant. Genentech (Roche) – Consultant. Gilead Sciences – Consultant. GlaxoSmithKline – Consultant. Gossamer Bio – Consultant. Imhotex – Consultant. Index Pharmaceuticals – Consultant. Innovation Pharmaceuticals – Consultant. Inotrem – Consultant. Janssen R&D – Consultant, Grant/Research Support, speaking fees. Kaleido – Consultant. Kallyope – Consultant. Merck & Co., Inc., Rahway, NJ, USA – Consultant. Microba – Consultant. Mobius Care – Consultant. Morphic Therapeutics – Consultant. MRM Health – Consultant. Nexus Therapeutics – Consultant. Nimbus Discovery – Consultant. Odyssey Therapeutics – Consultant. Pfizer – Consultant, Grant/Research Support, speaking fees. Progenity – Consultant. Prometheus Biosciences – Consultant. Prometheus Laboratories – Consultant. Protagonist Therapeutics – Consultant. Q32 Bio – Consultant. Rasayana Therapeutics – Consultant. Recludix Therapeutics – Consultant. Reistone Biopharma – Consultant. Sanofi – Consultant. Spyre Therapeutics – Consultant. Sun Pharma – Consultant. Surrozen – Consultant. Takeda – Consultant, Grant/Research Support, speaking fees. Target RWE – Consultant. Teva – Consultant. Theravance Biopharma – Consultant, Grant/Research Support. TLL Pharmaceutical – Consultant. Tr1X – Consultant. Union Therapeutics – Consultant. Ventyx Biosciences – Consultant, Stock Options.
Sami Hoque: MSD – Grant/Research Support.
Brian G. Feagan: Abbvie – Advisory Committee/Board Member, Consultant, honoraria. Abivax – Consultant. Adiso – Consultant. AgomAb Therapeutics – Consultant. Allianthera – Consultant. Amgen – Consultant. AnaptysBio – Advisory Committee/Board Member, Consultant. Arena Pharma – Consultant. Atomwise – Consultant. Avoro Capital Advisors – Consultant. BioJamp – Consultant. Biora Therapeutics – Consultant. Blackbird Laboratories – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Boxer Capital – Consultant. Celgene/BMS – Advisory Committee/Board Member, Consultant. Connect BioPharma – Consultant, Stock-publicly held company(excluding mutual/index funds). Cytoki – Consultant. Disc Medicine – Consultant. Duality – Consultant. EcoR1Capital – Advisory Committee/Board Member, Consultant. Eli Lilly – Advisory Committee/Board Member, Consultant. EnGene – Stock-publicly held company(excluding mutual/index funds). Equillium – Consultant. Ermium – Consultant. Evida – Stock-privately held company. First Wave – Consultant. Forbion – Consultant. Galapagos – Consultant. Galen Atlantica – Consultant. Genentech/Roche – Advisory Committee/Board Member, Consultant. Gilead – Consultant. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. Gossamer Pharma – Consultant. Hinge Bio – Consultant. Imhotex – Consultant. Immunic Therapeutics – Consultant. Index Pharma – Consultant. Intercept – Consultant. JAKAcademy – Consultant. Janssen – Advisory Committee/Board Member, Consultant, honoraria; expert testimony. Japan Tobacco Inc. – Consultant. Kaleido Biosciences – Consultant. Klick Health – Consultant. Landos Biopharma – Consultant. Lenczner Slaght – Consultant. LifeSci Capital – Consultant. Lument AB – Consultant. Mage Biologics – Consultant. Merck – Advisory Committee/Board Member. Mestag – Consultant. Millennium – Consultant. MiroBio – Advisory Committee/Board Member, Consultant. Monte Rosa Therapeutics – Consultant. Morgan Lewis – Consultant. Morphic Therapeutics – Advisory Committee/Board Member, Consultant. Mylan – Consultant. Nexera – Consultant. Nexys Therapeutics – Consultant. Nimbus Therapeutics – Consultant. Odyssey – Consultant. OM Pharma – Consultant. OrbiMed – Consultant. Origo BioPharma – Advisory Committee/Board Member, Consultant. Orphagen – Consultant. Pandion Therapeutics – Consultant. Pendopharm – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, honoraria. Progenity – Consultant. Prometheus Therapeutics and Diagnostics (Merck & Co., Inc., Rahway, NJ, USA) – Consultant. Protagonist – Consultant. PTM Therapeutics – Consultant. Q32 Bio – Consultant. Rebiotix – Consultant. REDX Pharma – Advisory Committee/Board Member, Consultant. Roche – Consultant. Roivant/Televant – Consultant. Sandoz – Consultant. Sanofi – Advisory Committee/Board Member, Consultant. Seres Therapeutics – Consultant. Silverback Therapeutics – Consultant. Sobi – Consultant. Spyre Therapeutics – Consultant. Surrozen Inc. – Consultant. Synedgen – Consultant. Takeda – Advisory Committee/Board Member, Consultant, honoraria. Teva – Advisory Committee/Board Member, Consultant. Thelium – Consultant. Tigenix – Consultant. Tillotts – Consultant. Triastek – Consultant. Ventyx – Consultant.
Mark Yen: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. – Employee, Stock Options.
Bin Dong: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. – Employee, Stock Options.
Wen Zhou: Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. – Employee, Stock Options.
Silvio Danese: AbbVie – Consultant, Lecture fees. Alimentiv – Consultant. Allergan – Consultant. Amgen – Consultant, Lecture fees. AstraZeneca – Consultant. Athos Therapeutics – Consultant. Biogen – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Celltrion – Consultant. Eli Lilly – Consultant. Enthera – Consultant. F. Hoffmann-La Roche Ltd – Consultant. Ferring Pharmaceuticals Inc. – Consultant, Lecture fees. Gilead – Consultant, Lecture fees. Hospira – Consultant. Inotrem – Consultant. Johnson & Johnson – Consultant, Lecture fees. MSD – Consultant. Mundipharma – Consultant. Mylan – Consultant, Lecture fees. Pfizer – Consultant, Lecture fees. Sandoz – Consultant. Sublimity Therapeutics – Consultant. Takeda – Consultant, Lecture fees. TiGenix – Consultant. UCB Inc. – Consultant. Vifor (International) Ltd. – Consultant.
Bruce E. Sands, MD, MS, FACG1, Sami Hoque, MD, PhD2, Brian G. Feagan, MD3, Mark Yen, 4, Bin Dong, 4, Wen Zhou, 4, Silvio Danese, MD, PhD5. P3194 - Efficacy and Safety of Tulisokibart Maintenance Treatment in Tulisokibart Induction, Re-induction, and Delayed Induction Responders: Open-Label Extension of the ARTEMIS-UC Trial in Patients With Ulcerative Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
