Monday Poster Session
Category: IBD
P3178 - Patient-Level Dynamics of Clinical Response to Mirikizumab and Its Impact on Quality of Life: Analysis from the Vivid-1 Trial in Crohn’s Disease
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- AV
Aisha Vadhariya
Eli Lilly and Company
Indianapolis, IN
Presenting Author(s)
Stefan Schreiber, 1, Aisha Vadhariya, 2, David B.. Clemow, PhD2, Guanglei Yu, PhD3, Laurie Keefer, PhD, FACG4, Ashwin Ananthakrishnan, 5, Geert R. D’Haens, MD, PhD6, Gursimran Kochhar, MD7, Huaiyu Zang, 8, Richard E.. Moses, DO, JD2, Parakkal Deepak, 9
1Department of Internal Medicine I, Kiel University, Kiel, Schleswig-Holstein, Germany; 2Eli Lilly and Company, Indianapolis, IN; 3Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 4Icahn School of Medicine at Mount Sinai, New York, NY; 5Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA; 6Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 7Division of Gastroenterology, Hepatology, and Nutrition, Allegheny Health Network, Pittsburgh, PA; 8Tigermed-BDM Inc., Somerset, NJ; 9Department of Medicine, Washington University School of Medicine, St. Louis, MO
Introduction: The efficacy of mirikizumab (MIRI), an anti-IL-23p19 monoclonal antibody, has been demonstrated in patients with moderately-to-severely active Crohn’s disease (CD) in the phase 3 VIVID-1 study (NCT03926130). Previously published patient-level data identified distinct responder populations with varying response dynamics. The Super Responder group with a fast robust response was generally linked to a higher proportion of patients achieving Week (W)52 clinical and endoscopic outcomes improvement. We evaluated improvements in patient-reported outcomes (PROs) and quality of life (QoL) among patients with CD with different patterns of response to MIRI.
Methods: The VIVID-1 study design was previously reported. This post-hoc analysis used a Growth Mixture Model to identify distinct MIRI patient clusters based on change from baseline in Crohn’s Disease Activity Index (CDAI). Outcomes evaluated across trajectory groups included clinical remission by PRO, clinically meaningful improvement in fatigue, change from baseline in abdominal pain and stool frequency, and Inflammatory Bowel Disease Questionnaire (IBDQ) response and remission. These outcomes were assessed using descriptive statistics at W12 and W52. Missing data were imputed using non-responder imputation and a modified baseline observation carried forward approach.
Results: Four response trajectory groups for MIRI were differentiated by early response and robustness of symptom resolution: Super Responder, Responder, Delayed Responder, and Non/Incomplete Responder. Achievement of clinical remission by PRO, fatigue improvement, and IBDQ response and remission at W12 and W52 was highest in Super Responders, followed by Responders, however high rates were also achieved among Delayed Responders (Table). In the Delayed Responder group, there was a large increase in proportion achieving clinical remission by PRO over time (W12, 31%; W52, 60%).
Discussion: In all three MIRI responder trajectory groups (70.3% of patients), QoL improvement was observed at W12 that was maintained at W52. The highest efficacy was seen among Super Responders, suggesting an early robust response to MIRI may be linked to greater long-term benefits. Efficacy gain between W12 and W52 in Delayed Responders reinforces the need to keep patients with response on MIRI.
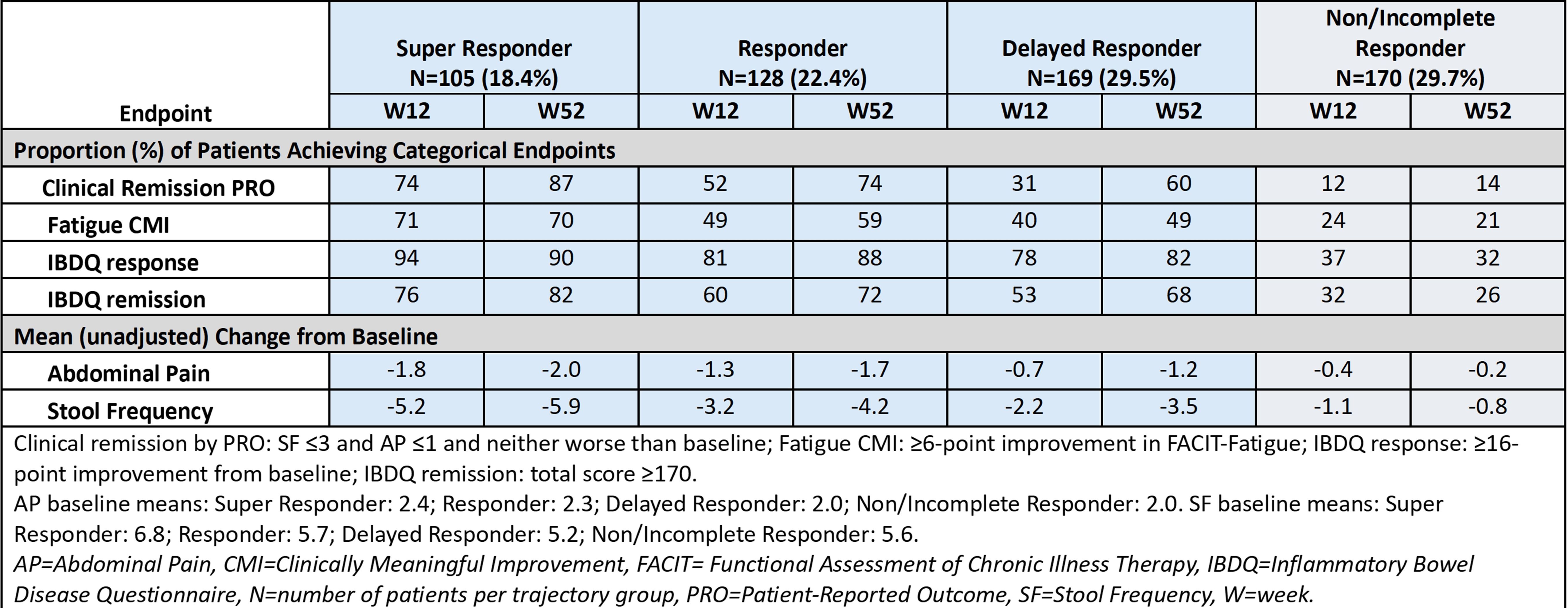
Figure: Patient-reported Outcomes by Trajectory Response Group
Disclosures:
Stefan Schreiber: AbbVie – Consultant, Speakers Bureau. AllergoSan – Consultant, Speakers Bureau. Amgen – Consultant, Speakers Bureau. Arena Pharmaceuticals – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr. Falk Pharma – Consultant, Speakers Bureau. Eli Lilly and Company – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galapagos NV – Consultant, Speakers Bureau. Genentech – Consultant, Speakers Bureau. Gilead Sciences – Consultant, Speakers Bureau. GSK Stiefel – Consultant, Speakers Bureau. I-Mab Biopharma – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Merck Sharp & Dohme – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau. Viatris – Consultant, Speakers Bureau.
Aisha Vadhariya: Eli Lilly and Company – Employee, Stock Options.
David Clemow: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Laurie Keefer: AbbVie – Consultant. Ardelyx – Consultant. Eli Lilly – Consultant. Janssen – Consultant. Pfizer – Consultant. Reckitt Health – Consultant. Trellus Health – Owner/Ownership Interest, Stock-publicly held company(excluding mutual/index funds).
Ashwin Ananthakrishnan: Takeda – Grant/Research Support.
Geert D’Haens: Abbvie – Advisor or Review Panel Member, Speakers Bureau. Abivax – Advisor or Review Panel Member. Agomab – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. AMT – Consultant. Anaptys Bio – Advisor or Review Panel Member. AstraZeneca – Consultant. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member. Exeliom – Consultant. Galapagos – Advisor or Review Panel Member. Glaxo Smith Kline – Advisor or Review Panel Member. Gossamerbio – Consultant. Immunic – Consultant. Index Pharmaceuticals – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member. Kaleido – Consultant. Merck – Advisor or Review Panel Member. Origo – Consultant. Pfizer – Consultant, Speakers Bureau. Polpharm – Advisor or Review Panel Member. Procise Diagnostics – Consultant. Progenity – Consultant. Prometheus Biosciences – Advisor or Review Panel Member. Protagonist Therapeutics – Consultant. Sanofi – Advisor or Review Panel Member. Seres Health – Advisory Committee/Board Member. Takeda – Advisor or Review Panel Member. Tillotts – Advisor or Review Panel Member.
Gursimran Kochhar: Boston Scientific Endoscopy – Consultant. Corvetas Research Foundation – Advisory Committee/Board Member. Digbi Health – Stock Options. Eli Lilly and Company – Advisory Committee/Board Member, Speakers Bureau. Exact Sciences – Consultant. GIE Medical – Advisory Committee/Board Member. Olympus Endoscopy – Consultant. Pentax Endoscopy – Consultant. Pharmacosmos – Advisory Committee/Board Member. Takeda – Consultant.
Huaiyu Zang: Eli Lilly and Company – Employee, Stock Options.
Richard Moses: Eli Lilly and Company – Employee, Stock Options.
Parakkal Deepak: Arena Pharmaceuticals – Grant/Research Support, Personal or other fees. Boehringer Ingelheim – Grant/Research Support, Personal or other fees. Bristol Myers Squibb/Celgene – Grant/Research Support, Personal or other fees. Janssen – Grant/Research Support, Personal or other fees. Pfizer – Grant/Research Support, Personal or other fees. Prometheus Biosciences – Grant/Research Support, Personal or other fees. Takeda – Grant/Research Support, Personal or other fees.
Stefan Schreiber, 1, Aisha Vadhariya, 2, David B.. Clemow, PhD2, Guanglei Yu, PhD3, Laurie Keefer, PhD, FACG4, Ashwin Ananthakrishnan, 5, Geert R. D’Haens, MD, PhD6, Gursimran Kochhar, MD7, Huaiyu Zang, 8, Richard E.. Moses, DO, JD2, Parakkal Deepak, 9. P3178 - Patient-Level Dynamics of Clinical Response to Mirikizumab and Its Impact on Quality of Life: Analysis from the Vivid-1 Trial in Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Internal Medicine I, Kiel University, Kiel, Schleswig-Holstein, Germany; 2Eli Lilly and Company, Indianapolis, IN; 3Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 4Icahn School of Medicine at Mount Sinai, New York, NY; 5Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA; 6Department of Gastroenterology, Amsterdam University Medical Center, Amsterdam, Noord-Holland, Netherlands; 7Division of Gastroenterology, Hepatology, and Nutrition, Allegheny Health Network, Pittsburgh, PA; 8Tigermed-BDM Inc., Somerset, NJ; 9Department of Medicine, Washington University School of Medicine, St. Louis, MO
Introduction: The efficacy of mirikizumab (MIRI), an anti-IL-23p19 monoclonal antibody, has been demonstrated in patients with moderately-to-severely active Crohn’s disease (CD) in the phase 3 VIVID-1 study (NCT03926130). Previously published patient-level data identified distinct responder populations with varying response dynamics. The Super Responder group with a fast robust response was generally linked to a higher proportion of patients achieving Week (W)52 clinical and endoscopic outcomes improvement. We evaluated improvements in patient-reported outcomes (PROs) and quality of life (QoL) among patients with CD with different patterns of response to MIRI.
Methods: The VIVID-1 study design was previously reported. This post-hoc analysis used a Growth Mixture Model to identify distinct MIRI patient clusters based on change from baseline in Crohn’s Disease Activity Index (CDAI). Outcomes evaluated across trajectory groups included clinical remission by PRO, clinically meaningful improvement in fatigue, change from baseline in abdominal pain and stool frequency, and Inflammatory Bowel Disease Questionnaire (IBDQ) response and remission. These outcomes were assessed using descriptive statistics at W12 and W52. Missing data were imputed using non-responder imputation and a modified baseline observation carried forward approach.
Results: Four response trajectory groups for MIRI were differentiated by early response and robustness of symptom resolution: Super Responder, Responder, Delayed Responder, and Non/Incomplete Responder. Achievement of clinical remission by PRO, fatigue improvement, and IBDQ response and remission at W12 and W52 was highest in Super Responders, followed by Responders, however high rates were also achieved among Delayed Responders (Table). In the Delayed Responder group, there was a large increase in proportion achieving clinical remission by PRO over time (W12, 31%; W52, 60%).
Discussion: In all three MIRI responder trajectory groups (70.3% of patients), QoL improvement was observed at W12 that was maintained at W52. The highest efficacy was seen among Super Responders, suggesting an early robust response to MIRI may be linked to greater long-term benefits. Efficacy gain between W12 and W52 in Delayed Responders reinforces the need to keep patients with response on MIRI.

Figure: Patient-reported Outcomes by Trajectory Response Group
Disclosures:
Stefan Schreiber: AbbVie – Consultant, Speakers Bureau. AllergoSan – Consultant, Speakers Bureau. Amgen – Consultant, Speakers Bureau. Arena Pharmaceuticals – Consultant, Speakers Bureau. Celltrion – Consultant, Speakers Bureau. Dr. Falk Pharma – Consultant, Speakers Bureau. Eli Lilly and Company – Consultant, Speakers Bureau. Ferring Pharmaceuticals – Consultant, Speakers Bureau. Galapagos NV – Consultant, Speakers Bureau. Genentech – Consultant, Speakers Bureau. Gilead Sciences – Consultant, Speakers Bureau. GSK Stiefel – Consultant, Speakers Bureau. I-Mab Biopharma – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Merck Sharp & Dohme – Consultant, Speakers Bureau. Pfizer – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Takeda – Consultant, Speakers Bureau. Viatris – Consultant, Speakers Bureau.
Aisha Vadhariya: Eli Lilly and Company – Employee, Stock Options.
David Clemow: Eli Lilly and Company – Employee, Stock Options.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Laurie Keefer: AbbVie – Consultant. Ardelyx – Consultant. Eli Lilly – Consultant. Janssen – Consultant. Pfizer – Consultant. Reckitt Health – Consultant. Trellus Health – Owner/Ownership Interest, Stock-publicly held company(excluding mutual/index funds).
Ashwin Ananthakrishnan: Takeda – Grant/Research Support.
Geert D’Haens: Abbvie – Advisor or Review Panel Member, Speakers Bureau. Abivax – Advisor or Review Panel Member. Agomab – Advisor or Review Panel Member. Alimentiv – Advisor or Review Panel Member. AMT – Consultant. Anaptys Bio – Advisor or Review Panel Member. AstraZeneca – Consultant. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member. Eli Lilly – Advisor or Review Panel Member. Exeliom – Consultant. Galapagos – Advisor or Review Panel Member. Glaxo Smith Kline – Advisor or Review Panel Member. Gossamerbio – Consultant. Immunic – Consultant. Index Pharmaceuticals – Advisor or Review Panel Member. Johnson & Johnson – Advisor or Review Panel Member. Kaleido – Consultant. Merck – Advisor or Review Panel Member. Origo – Consultant. Pfizer – Consultant, Speakers Bureau. Polpharm – Advisor or Review Panel Member. Procise Diagnostics – Consultant. Progenity – Consultant. Prometheus Biosciences – Advisor or Review Panel Member. Protagonist Therapeutics – Consultant. Sanofi – Advisor or Review Panel Member. Seres Health – Advisory Committee/Board Member. Takeda – Advisor or Review Panel Member. Tillotts – Advisor or Review Panel Member.
Gursimran Kochhar: Boston Scientific Endoscopy – Consultant. Corvetas Research Foundation – Advisory Committee/Board Member. Digbi Health – Stock Options. Eli Lilly and Company – Advisory Committee/Board Member, Speakers Bureau. Exact Sciences – Consultant. GIE Medical – Advisory Committee/Board Member. Olympus Endoscopy – Consultant. Pentax Endoscopy – Consultant. Pharmacosmos – Advisory Committee/Board Member. Takeda – Consultant.
Huaiyu Zang: Eli Lilly and Company – Employee, Stock Options.
Richard Moses: Eli Lilly and Company – Employee, Stock Options.
Parakkal Deepak: Arena Pharmaceuticals – Grant/Research Support, Personal or other fees. Boehringer Ingelheim – Grant/Research Support, Personal or other fees. Bristol Myers Squibb/Celgene – Grant/Research Support, Personal or other fees. Janssen – Grant/Research Support, Personal or other fees. Pfizer – Grant/Research Support, Personal or other fees. Prometheus Biosciences – Grant/Research Support, Personal or other fees. Takeda – Grant/Research Support, Personal or other fees.
Stefan Schreiber, 1, Aisha Vadhariya, 2, David B.. Clemow, PhD2, Guanglei Yu, PhD3, Laurie Keefer, PhD, FACG4, Ashwin Ananthakrishnan, 5, Geert R. D’Haens, MD, PhD6, Gursimran Kochhar, MD7, Huaiyu Zang, 8, Richard E.. Moses, DO, JD2, Parakkal Deepak, 9. P3178 - Patient-Level Dynamics of Clinical Response to Mirikizumab and Its Impact on Quality of Life: Analysis from the Vivid-1 Trial in Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
