Monday Poster Session
Category: IBD
P3175 - Comparative Safety of Ustekinumab vs Anti-TNF Therapy During Pregnancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Ali Emre Bardak, MD
St. Elizabeth's Medical Center, Boston University School of Medicine
Boston, MA
Presenting Author(s)
Ali Emre Bardak, MD1, Humza Saeed, 2, Gizem Teker, MD3, Sonia Friedman, MD4, Saqr Alsakarneh, MD, MS5, Stefan Mitev, MD6
1St. Elizabeth's Medical Center, Boston University School of Medicine, Boston, MA; 2Rawalpindi Medical University, Rawalpindi, Punjab, Pakistan; 3Istanbul University, Istanbul, Istanbul, Turkey; 4Tufts Medical Center, Boston, MA; 5Mayo Clinic, Kansas City, MO; 6University Hospital St Ivan Rilski, Sofiya, Sofiya, Bulgaria
Introduction: Inflammatory bowel disease (IBD) frequently affects women of reproductive age. While the safety of anti-tumor necrosis factor (anti-TNF) agents during pregnancy is well established, data on the safety of ustekinumab remain limited. We aimed to compare the safety of ustekinumab versus anti-TNF therapy in pregnant patients with IBD in terms of pregnancy and neonatal outcomes.
Methods: We systematically searched PubMed, Embase, and Cochrane databases through November 2024. Studies comparing ustekinumab and anti-TNF agents in pregnant patients with IBD and reporting key pregnancy or neonatal outcomes were included. Odds ratio (OR) was used as the effect measure. Pooled analyses were performed using random-effects models.
Results: Four studies, encompassing 3,308 pregnancies (592 ustekinumab, 2,716 anti-TNF) were included. The general characteristics of the studies included in the meta-analysis are presented in Figure 1. The majority of patients (2,914; 88.2%) had Crohn’s disease, and median disease duration ranged from 6.5 to 14 years. There was no significant difference between ustekinumab and anti-TNF therapy in live birth rates (67.2% vs 67.7%; OR 0.73, 95% CI 0.39-1.37), spontaneous abortion rates (5.9% vs 4.2%; OR 1.51, 95% CI 0.74-3.36), preterm delivery rates (6.6% vs 7.4%; OR 0.50, 95% CI 0.15-1.61), low birth weight rates (4.6% vs 7.1%; OR 0.68, 95% CI 0.23-1.98), and cesarean section rates (30.0% vs 30.1%; OR 1.11, 95% CI 0.85-1.45). Additional details regarding the pooled outcomes are presented in Figure 2.
Discussion: No significant differences in major pregnancy and neonatal outcomes were found between ustekinumab-exposed and anti-TNF-exposed pregnancies. These findings suggest the use of ustekinumab during pregnancy is comparable with anti-TNF therapy and can be considered safe. Nevertheless, it should be acknowledged that the data are still limited, and further studies are needed.
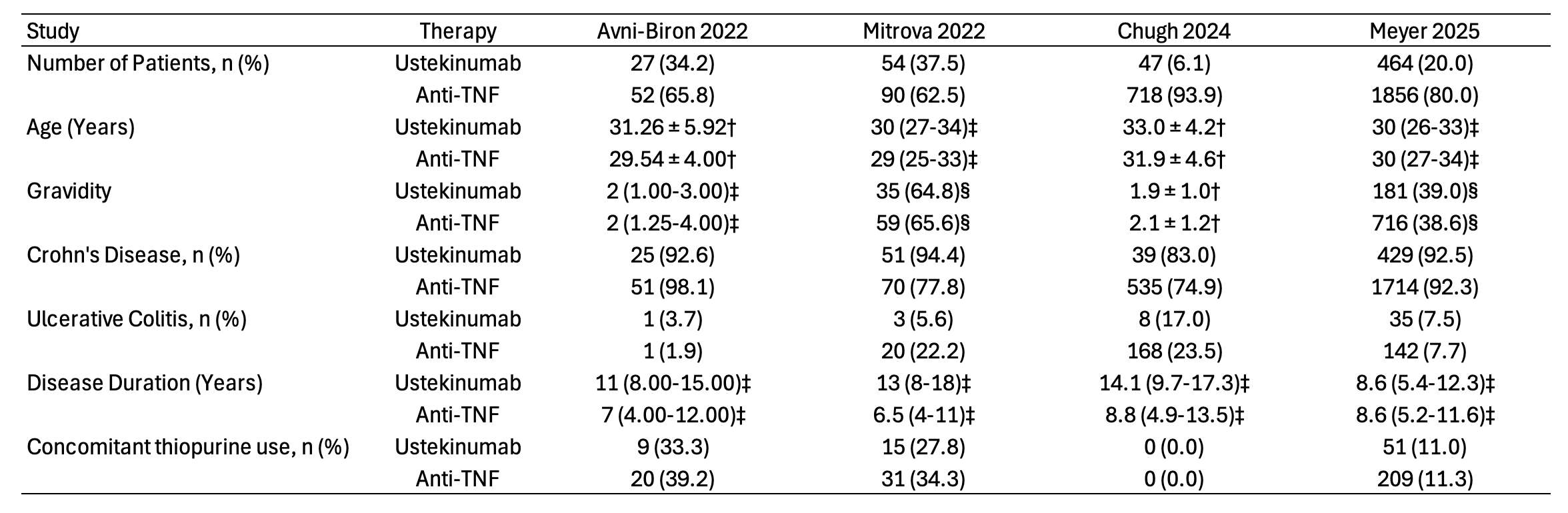
Figure: Baseline characteristics of patients in the included studies. Values are presented as mean ± standard deviation †; median (interquartile range) ‡; first pregnancy number (percentage) §. Anti-TNF = Anti-tumor necrosis factor.
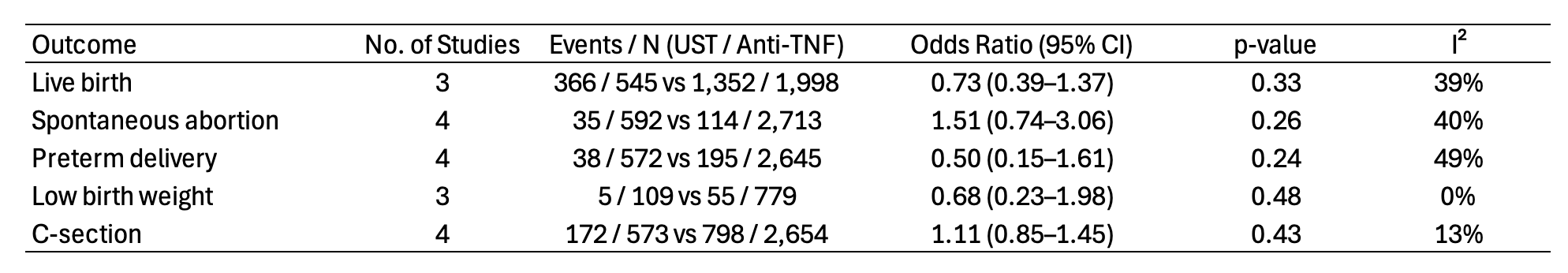
Figure: Pooled pregnancy and neonatal outcomes comparing ustekinumab versus anti-tumor necrosis factor therapy. UST = Ustekinumab; Anti-TNF = Anti-tumor necrosis factor.
Disclosures:
Ali Emre Bardak indicated no relevant financial relationships.
Humza Saeed indicated no relevant financial relationships.
Gizem Teker indicated no relevant financial relationships.
Sonia Friedman indicated no relevant financial relationships.
Saqr Alsakarneh indicated no relevant financial relationships.
Stefan Mitev indicated no relevant financial relationships.
Ali Emre Bardak, MD1, Humza Saeed, 2, Gizem Teker, MD3, Sonia Friedman, MD4, Saqr Alsakarneh, MD, MS5, Stefan Mitev, MD6. P3175 - Comparative Safety of Ustekinumab vs Anti-TNF Therapy During Pregnancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1St. Elizabeth's Medical Center, Boston University School of Medicine, Boston, MA; 2Rawalpindi Medical University, Rawalpindi, Punjab, Pakistan; 3Istanbul University, Istanbul, Istanbul, Turkey; 4Tufts Medical Center, Boston, MA; 5Mayo Clinic, Kansas City, MO; 6University Hospital St Ivan Rilski, Sofiya, Sofiya, Bulgaria
Introduction: Inflammatory bowel disease (IBD) frequently affects women of reproductive age. While the safety of anti-tumor necrosis factor (anti-TNF) agents during pregnancy is well established, data on the safety of ustekinumab remain limited. We aimed to compare the safety of ustekinumab versus anti-TNF therapy in pregnant patients with IBD in terms of pregnancy and neonatal outcomes.
Methods: We systematically searched PubMed, Embase, and Cochrane databases through November 2024. Studies comparing ustekinumab and anti-TNF agents in pregnant patients with IBD and reporting key pregnancy or neonatal outcomes were included. Odds ratio (OR) was used as the effect measure. Pooled analyses were performed using random-effects models.
Results: Four studies, encompassing 3,308 pregnancies (592 ustekinumab, 2,716 anti-TNF) were included. The general characteristics of the studies included in the meta-analysis are presented in Figure 1. The majority of patients (2,914; 88.2%) had Crohn’s disease, and median disease duration ranged from 6.5 to 14 years. There was no significant difference between ustekinumab and anti-TNF therapy in live birth rates (67.2% vs 67.7%; OR 0.73, 95% CI 0.39-1.37), spontaneous abortion rates (5.9% vs 4.2%; OR 1.51, 95% CI 0.74-3.36), preterm delivery rates (6.6% vs 7.4%; OR 0.50, 95% CI 0.15-1.61), low birth weight rates (4.6% vs 7.1%; OR 0.68, 95% CI 0.23-1.98), and cesarean section rates (30.0% vs 30.1%; OR 1.11, 95% CI 0.85-1.45). Additional details regarding the pooled outcomes are presented in Figure 2.
Discussion: No significant differences in major pregnancy and neonatal outcomes were found between ustekinumab-exposed and anti-TNF-exposed pregnancies. These findings suggest the use of ustekinumab during pregnancy is comparable with anti-TNF therapy and can be considered safe. Nevertheless, it should be acknowledged that the data are still limited, and further studies are needed.

Figure: Baseline characteristics of patients in the included studies. Values are presented as mean ± standard deviation †; median (interquartile range) ‡; first pregnancy number (percentage) §. Anti-TNF = Anti-tumor necrosis factor.

Figure: Pooled pregnancy and neonatal outcomes comparing ustekinumab versus anti-tumor necrosis factor therapy. UST = Ustekinumab; Anti-TNF = Anti-tumor necrosis factor.
Disclosures:
Ali Emre Bardak indicated no relevant financial relationships.
Humza Saeed indicated no relevant financial relationships.
Gizem Teker indicated no relevant financial relationships.
Sonia Friedman indicated no relevant financial relationships.
Saqr Alsakarneh indicated no relevant financial relationships.
Stefan Mitev indicated no relevant financial relationships.
Ali Emre Bardak, MD1, Humza Saeed, 2, Gizem Teker, MD3, Sonia Friedman, MD4, Saqr Alsakarneh, MD, MS5, Stefan Mitev, MD6. P3175 - Comparative Safety of Ustekinumab vs Anti-TNF Therapy During Pregnancy in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

