Monday Poster Session
Category: GI Bleeding
P3083 - Hemostatic Powders Are Equivalent to Standard Therapy in Benign Upper GI Bleeding and Superior in Tumor-Related Bleeding: A Systematic Review and Meta-Analysis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Ashesh Das, MBBS
KPC Medical College and Hospital , Kolkata, India
Kolkata, West Bengal, India
Presenting Author(s)
Ashesh Das, MBBS1, Urvashi Bharia, 2, Fazia Khattak, 3, Venkata Dileep Kumar Veldi, MBBS4, Daniel Razgonyaev, 5, Scott Tenner, MD6
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Lokmanya Tilak Municipal Medical College and General Hospital, Mumbai, Navi Mumbai, Maharashtra, India; 3Khyber Medical College, Peshawar, North-West Frontier, Pakistan; 4Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 5SUNY Downstate Medical Center, Brooklyn, NY; 6State University of New York, Downstate, Brooklyn, NY
Introduction: Despite advances in methods of controlling active non-variceal upper gastrointestinal bleeding (UGIB), approximately 10-20% of patients treated require repeat endoscopy within 48 hours due to persistent bleeding and/or rebleeding after initial hemostasis. Contact-free mineral hemostatic powders can be deployed in seconds and might circumvent technical failures, yet their aggregate efficacy and whether it varies by bleed etiology remains unclear. We therefore pooled all contemporary randomized trials, stratifying a priori into benign non-variceal versus tumor-related UGIB, to clarify where powders add value.
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified Randomized Controlled Trials (RCTs) comparing Hemostatic Powder to Standard Therapy in Upper GI Bleed and Malignant Bleed through May 2025. Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Across 255 benign NVUGIB episodes (Baracat 2019 + Jung 2023) topical powder achieved immediate haemostasis in 90 % vs 87 % with guideline therapy (RR 1.04, 95 % CI 0.95–1.13; I² = 0 %) and the 30-day re-bleed rate was identical (10 % vs 10 %; RR 1.08, 0.52–2.23; I² = 0 %).In sharp contrast, the 111-patient malignant-bleeding RCT (Pittayanon 2023) showed 100 % first-pass haemostasis versus 69 % (RR 1.45, 1.20–1.75) and cut re-bleeding from 22 % to 6 % (RR 0.26, 0.08–0.80), yielding an absolute risk reduction of 16 % and NNT = 6.Interaction testing confirmed significant effect-modification between subgroups for both haemostasis (χ² = 10.2, P = 0.001) and re-bleeding (χ² = 4.3, P = 0.04), fully accounting for the overall heterogeneity (I² 82 % and 61 %, respectively). Thirty-day mortality was low and comparable (11/179 vs 8/182; RR 1.35, 0.58–3.14).
Discussion: Topical powder therapy proved non-inferior to standard care in routine peptic-ulcer bleeding but reduced tumor-bleed re-bleeding by 74 % (NNT = 6), with zero formulation-related adverse events. The dramatic oncologic benefit and equivalent benign performance position TC-325 as a high-value first-line option whenever malignancy underlies hemorrhage, while remaining a safe alternative in benign disease. Future multicenter RCTs should target malignancy-specific algorithms.
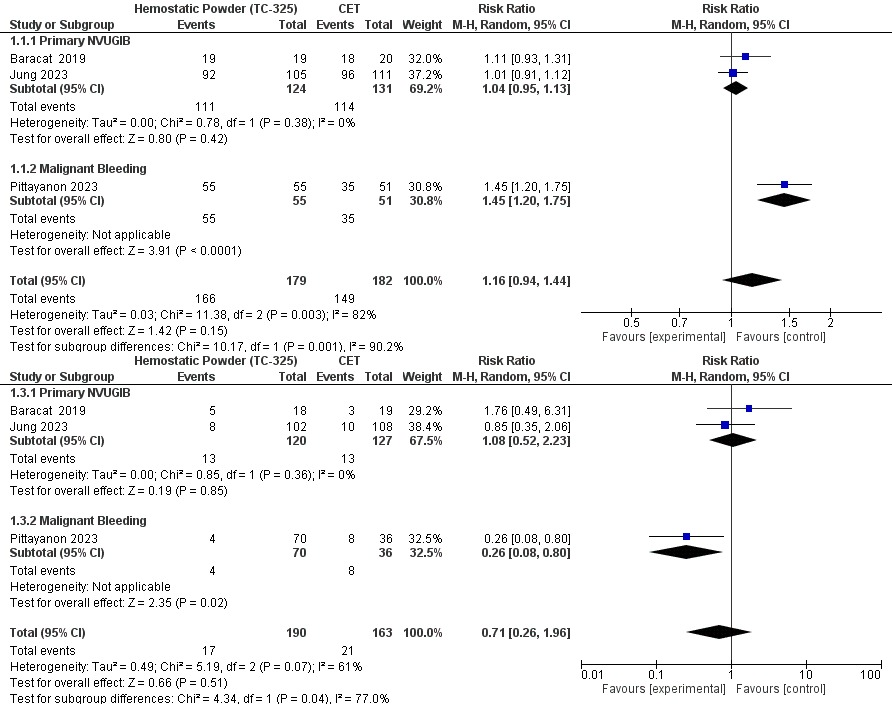
Figure: Forest Plot Showing Re-Bleeding and Initial Hemostasis
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Urvashi Bharia indicated no relevant financial relationships.
Fazia Khattak indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Daniel Razgonyaev indicated no relevant financial relationships.
Scott Tenner indicated no relevant financial relationships.
Ashesh Das, MBBS1, Urvashi Bharia, 2, Fazia Khattak, 3, Venkata Dileep Kumar Veldi, MBBS4, Daniel Razgonyaev, 5, Scott Tenner, MD6. P3083 - Hemostatic Powders Are Equivalent to Standard Therapy in Benign Upper GI Bleeding and Superior in Tumor-Related Bleeding: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Lokmanya Tilak Municipal Medical College and General Hospital, Mumbai, Navi Mumbai, Maharashtra, India; 3Khyber Medical College, Peshawar, North-West Frontier, Pakistan; 4Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 5SUNY Downstate Medical Center, Brooklyn, NY; 6State University of New York, Downstate, Brooklyn, NY
Introduction: Despite advances in methods of controlling active non-variceal upper gastrointestinal bleeding (UGIB), approximately 10-20% of patients treated require repeat endoscopy within 48 hours due to persistent bleeding and/or rebleeding after initial hemostasis. Contact-free mineral hemostatic powders can be deployed in seconds and might circumvent technical failures, yet their aggregate efficacy and whether it varies by bleed etiology remains unclear. We therefore pooled all contemporary randomized trials, stratifying a priori into benign non-variceal versus tumor-related UGIB, to clarify where powders add value.
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified Randomized Controlled Trials (RCTs) comparing Hemostatic Powder to Standard Therapy in Upper GI Bleed and Malignant Bleed through May 2025. Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Across 255 benign NVUGIB episodes (Baracat 2019 + Jung 2023) topical powder achieved immediate haemostasis in 90 % vs 87 % with guideline therapy (RR 1.04, 95 % CI 0.95–1.13; I² = 0 %) and the 30-day re-bleed rate was identical (10 % vs 10 %; RR 1.08, 0.52–2.23; I² = 0 %).In sharp contrast, the 111-patient malignant-bleeding RCT (Pittayanon 2023) showed 100 % first-pass haemostasis versus 69 % (RR 1.45, 1.20–1.75) and cut re-bleeding from 22 % to 6 % (RR 0.26, 0.08–0.80), yielding an absolute risk reduction of 16 % and NNT = 6.Interaction testing confirmed significant effect-modification between subgroups for both haemostasis (χ² = 10.2, P = 0.001) and re-bleeding (χ² = 4.3, P = 0.04), fully accounting for the overall heterogeneity (I² 82 % and 61 %, respectively). Thirty-day mortality was low and comparable (11/179 vs 8/182; RR 1.35, 0.58–3.14).
Discussion: Topical powder therapy proved non-inferior to standard care in routine peptic-ulcer bleeding but reduced tumor-bleed re-bleeding by 74 % (NNT = 6), with zero formulation-related adverse events. The dramatic oncologic benefit and equivalent benign performance position TC-325 as a high-value first-line option whenever malignancy underlies hemorrhage, while remaining a safe alternative in benign disease. Future multicenter RCTs should target malignancy-specific algorithms.

Figure: Forest Plot Showing Re-Bleeding and Initial Hemostasis
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Urvashi Bharia indicated no relevant financial relationships.
Fazia Khattak indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Daniel Razgonyaev indicated no relevant financial relationships.
Scott Tenner indicated no relevant financial relationships.
Ashesh Das, MBBS1, Urvashi Bharia, 2, Fazia Khattak, 3, Venkata Dileep Kumar Veldi, MBBS4, Daniel Razgonyaev, 5, Scott Tenner, MD6. P3083 - Hemostatic Powders Are Equivalent to Standard Therapy in Benign Upper GI Bleeding and Superior in Tumor-Related Bleeding: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
