Monday Poster Session
Category: General Endoscopy
P3004 - Adverse Events Associated With Over-the-Scope Clips: A 10-Year Retrospective Review from the FDA MAUDE Database
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- OA
Osama Alshakhatreh, MD (he/him/his)
Albany Medical Center
Norfolk, VA
Presenting Author(s)
Osama Alshakhatreh, MD, Ibrahim Mohammed, MD, Tarick Ahmad, BS, Shunsa Tarar, DO, Mohsin Chundrigar, MBBS, Seth Richter, MD, FACG
Albany Medical Center, Albany, NY
Introduction: Over-the-scope clips (OTSCs) are valuable tools in gastrointestinal procedures for hemostasis, closure of perforations, or tissue resection. While effective, OTSCs have been associated with adverse events in post-marketing surveillance. This study analyzes reports from the FDA Manufacturer and User Facility Device Experience (MAUDE) database to identify the frequency and nature of OTSC-related complications between 2015 and 2025.
Methods: We conducted a retrospective review of MAUDE reports related to OTSC systems, including both Ovesco and Padlock devices. Reports were reviewed manually and categorized as device-related or patient-related adverse events using a standardized classification system. Duplicate entries were excluded. Reports with unclear narratives were classified as "other." Frequencies and percentages were calculated for each event type.
Results: A total of 149 device-related and 123 patient-related adverse events were identified. The most common device-related issues were clip deployment failure (22%), clip separation problems (10%), and defective components (12%). Patient-related adverse events included perforation (58%) and bleeding (18%), followed by pain and fistula formation. Perforations were reported more frequently with full-thickness resection devices (FTRD) than with OTSC-only systems (86% vs. 32%). Death was infrequently reported and occurred only in patients with significant comorbidities. A detailed summary of these events is shown in Table (1).
Discussion: This 10-year review of OTSC-related adverse events highlights common complications including deployment failure, device defects, and serious outcomes such as perforation and bleeding. The higher perforation rate with FTRD highlights the importance of procedural planning and patient selection. These findings support the need for continued post-market surveillance, standardized reporting, and enhanced practitioner training to minimize risks. Future prospective studies are warranted to better identify predictive factors for complications in OTSC-based interventions.
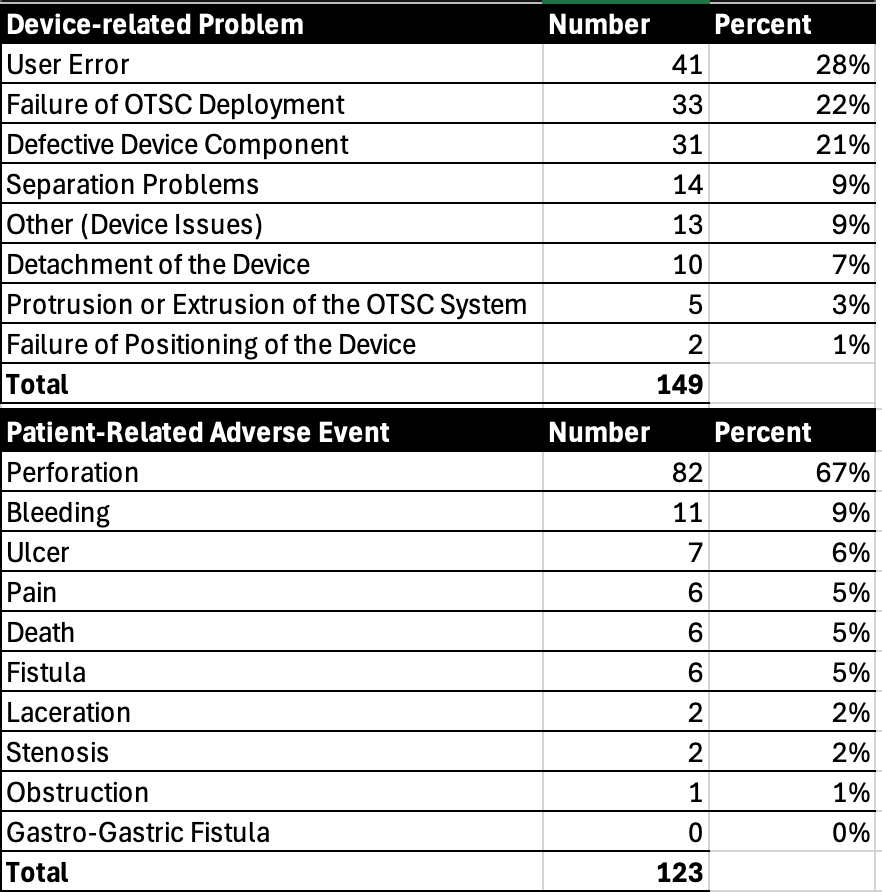
Figure: Table (1). Summary of Device- and Patient-Related Adverse Events Associated with Over-the-Scope Clip (OTSC) Systems Reported in the FDA MAUDE Database, 2015–2025
Disclosures:
Osama Alshakhatreh indicated no relevant financial relationships.
Ibrahim Mohammed indicated no relevant financial relationships.
Tarick Ahmad indicated no relevant financial relationships.
Shunsa Tarar indicated no relevant financial relationships.
Mohsin Chundrigar indicated no relevant financial relationships.
Seth Richter indicated no relevant financial relationships.
Osama Alshakhatreh, MD, Ibrahim Mohammed, MD, Tarick Ahmad, BS, Shunsa Tarar, DO, Mohsin Chundrigar, MBBS, Seth Richter, MD, FACG. P3004 - Adverse Events Associated With Over-the-Scope Clips: A 10-Year Retrospective Review from the FDA MAUDE Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Albany Medical Center, Albany, NY
Introduction: Over-the-scope clips (OTSCs) are valuable tools in gastrointestinal procedures for hemostasis, closure of perforations, or tissue resection. While effective, OTSCs have been associated with adverse events in post-marketing surveillance. This study analyzes reports from the FDA Manufacturer and User Facility Device Experience (MAUDE) database to identify the frequency and nature of OTSC-related complications between 2015 and 2025.
Methods: We conducted a retrospective review of MAUDE reports related to OTSC systems, including both Ovesco and Padlock devices. Reports were reviewed manually and categorized as device-related or patient-related adverse events using a standardized classification system. Duplicate entries were excluded. Reports with unclear narratives were classified as "other." Frequencies and percentages were calculated for each event type.
Results: A total of 149 device-related and 123 patient-related adverse events were identified. The most common device-related issues were clip deployment failure (22%), clip separation problems (10%), and defective components (12%). Patient-related adverse events included perforation (58%) and bleeding (18%), followed by pain and fistula formation. Perforations were reported more frequently with full-thickness resection devices (FTRD) than with OTSC-only systems (86% vs. 32%). Death was infrequently reported and occurred only in patients with significant comorbidities. A detailed summary of these events is shown in Table (1).
Discussion: This 10-year review of OTSC-related adverse events highlights common complications including deployment failure, device defects, and serious outcomes such as perforation and bleeding. The higher perforation rate with FTRD highlights the importance of procedural planning and patient selection. These findings support the need for continued post-market surveillance, standardized reporting, and enhanced practitioner training to minimize risks. Future prospective studies are warranted to better identify predictive factors for complications in OTSC-based interventions.

Figure: Table (1). Summary of Device- and Patient-Related Adverse Events Associated with Over-the-Scope Clip (OTSC) Systems Reported in the FDA MAUDE Database, 2015–2025
Disclosures:
Osama Alshakhatreh indicated no relevant financial relationships.
Ibrahim Mohammed indicated no relevant financial relationships.
Tarick Ahmad indicated no relevant financial relationships.
Shunsa Tarar indicated no relevant financial relationships.
Mohsin Chundrigar indicated no relevant financial relationships.
Seth Richter indicated no relevant financial relationships.
Osama Alshakhatreh, MD, Ibrahim Mohammed, MD, Tarick Ahmad, BS, Shunsa Tarar, DO, Mohsin Chundrigar, MBBS, Seth Richter, MD, FACG. P3004 - Adverse Events Associated With Over-the-Scope Clips: A 10-Year Retrospective Review from the FDA MAUDE Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
