Monday Poster Session
Category: Functional Bowel Disease
P2926 - A Randomized Controlled Trial of a Digital Cognitive Behavioral Therapy Application for Irritable Bowel Syndrome
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- MS
Mai Sedki, MD, MPH (she/her/hers)
Stanford University School of Medicine
Palo Alto, CA
Presenting Author(s)
Mai Sedki, MD, MPH1, Meredith Craven, PhD1, Linda Nguyen, MD2, Irene Sonu, MD1
1Stanford University School of Medicine, Palo Alto, CA; 2Stanford University School of Medicine, Stanford, CA
Introduction: Irritable bowel syndrome (IBS) is a common disorder of gut-brain interaction with multifactorial pathophysiology that significantly impairs quality of life. Cognitive behavioral therapy (CBT) has demonstrated efficacy for symptom reduction but is limited by access barriers. This study evaluated the efficacy of a CBT-based smartphone application in reducing IBS symptoms compared to treatment as usual (TAU) in a randomized controlled trial.
Methods: We conducted a single-blind randomized controlled trial to evaluate the effectiveness of a CBT smartphone application in reducing IBS symptom burden. Adults meeting Rome IV criteria for IBS were randomized 1:1 to receive treatment-as-usual (TAU) or TAU plus an 8-week intervention via a CBT-based smartphone application. The primary outcome was change in IBS Symptom Severity Scale (IBS-SSS) score at 8 weeks. Secondary outcomes included changes in perceived stress (PSQ), work and social adjustment (WSAS), mood (HADS), and IBS-SSS scores at 24 weeks. Outcomes were assessed at baseline, week 8, and week 24.
Results: Of 18 enrolled participants (median age 35.5 years, 72% female), 15 completed follow-up. Baseline characteristics were generally balanced between groups, although the CBT app group had significantly higher baseline perceived stress (median PSQ 32 vs. 21, p=0.0167) (Table 1A). At week 8, the CBT app group showed a greater median reduction in IBS-SSS score (-108 [IQR -149, -2]) than TAU (-56.5 [IQR -86, -2]), though the difference was not statistically significant (p=0.479). At week 24, both groups demonstrated sustained symptom improvement, with a larger reduction in the CBT app group (-55 vs. -22.5) (Table 1B). These trends are illustrated in Figure 1, which depicts IBS-SSS scores over time. No significant between-group differences were observed in changes in HADS, PSQ, or WSAS scores. No adverse events were reported.
Discussion: This pilot randomized trial demonstrates that a mobile-delivered CBT program for IBS is both feasible and safe, with clinically meaningful symptom improvements and reduced perceived stress observed over 8 and 24 weeks. Although differences between intervention and control groups did not reach statistical significance, consistent trends favoring the digital intervention support the need for further evaluation in a larger, adequately powered study. Mobile CBT may represent a promising, scalable adjunct to usual care in IBS management.
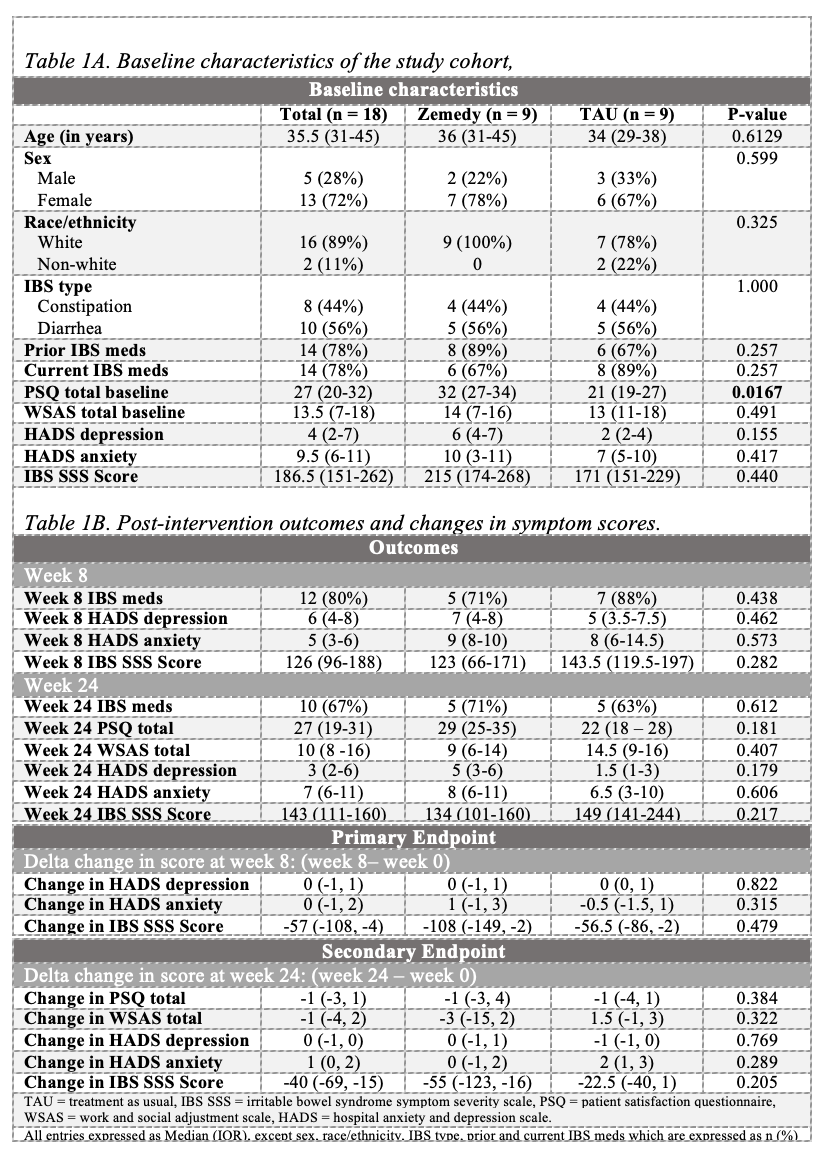
Figure: Baseline Characteristics of study cohort & Post-intervention outcomes and changes in symptom scores.
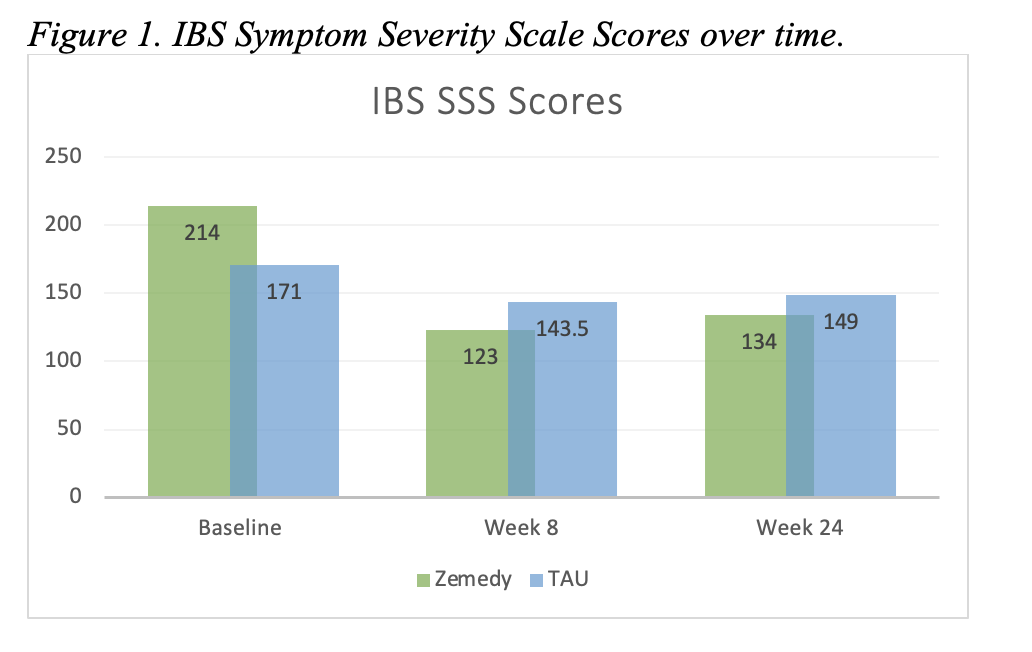
Figure: IBS Symptom Severity Scale Scores over time.
Disclosures:
Mai Sedki indicated no relevant financial relationships.
Meredith Craven indicated no relevant financial relationships.
Linda Nguyen indicated no relevant financial relationships.
Irene Sonu indicated no relevant financial relationships.
Mai Sedki, MD, MPH1, Meredith Craven, PhD1, Linda Nguyen, MD2, Irene Sonu, MD1. P2926 - A Randomized Controlled Trial of a Digital Cognitive Behavioral Therapy Application for Irritable Bowel Syndrome, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Stanford University School of Medicine, Palo Alto, CA; 2Stanford University School of Medicine, Stanford, CA
Introduction: Irritable bowel syndrome (IBS) is a common disorder of gut-brain interaction with multifactorial pathophysiology that significantly impairs quality of life. Cognitive behavioral therapy (CBT) has demonstrated efficacy for symptom reduction but is limited by access barriers. This study evaluated the efficacy of a CBT-based smartphone application in reducing IBS symptoms compared to treatment as usual (TAU) in a randomized controlled trial.
Methods: We conducted a single-blind randomized controlled trial to evaluate the effectiveness of a CBT smartphone application in reducing IBS symptom burden. Adults meeting Rome IV criteria for IBS were randomized 1:1 to receive treatment-as-usual (TAU) or TAU plus an 8-week intervention via a CBT-based smartphone application. The primary outcome was change in IBS Symptom Severity Scale (IBS-SSS) score at 8 weeks. Secondary outcomes included changes in perceived stress (PSQ), work and social adjustment (WSAS), mood (HADS), and IBS-SSS scores at 24 weeks. Outcomes were assessed at baseline, week 8, and week 24.
Results: Of 18 enrolled participants (median age 35.5 years, 72% female), 15 completed follow-up. Baseline characteristics were generally balanced between groups, although the CBT app group had significantly higher baseline perceived stress (median PSQ 32 vs. 21, p=0.0167) (Table 1A). At week 8, the CBT app group showed a greater median reduction in IBS-SSS score (-108 [IQR -149, -2]) than TAU (-56.5 [IQR -86, -2]), though the difference was not statistically significant (p=0.479). At week 24, both groups demonstrated sustained symptom improvement, with a larger reduction in the CBT app group (-55 vs. -22.5) (Table 1B). These trends are illustrated in Figure 1, which depicts IBS-SSS scores over time. No significant between-group differences were observed in changes in HADS, PSQ, or WSAS scores. No adverse events were reported.
Discussion: This pilot randomized trial demonstrates that a mobile-delivered CBT program for IBS is both feasible and safe, with clinically meaningful symptom improvements and reduced perceived stress observed over 8 and 24 weeks. Although differences between intervention and control groups did not reach statistical significance, consistent trends favoring the digital intervention support the need for further evaluation in a larger, adequately powered study. Mobile CBT may represent a promising, scalable adjunct to usual care in IBS management.

Figure: Baseline Characteristics of study cohort & Post-intervention outcomes and changes in symptom scores.

Figure: IBS Symptom Severity Scale Scores over time.
Disclosures:
Mai Sedki indicated no relevant financial relationships.
Meredith Craven indicated no relevant financial relationships.
Linda Nguyen indicated no relevant financial relationships.
Irene Sonu indicated no relevant financial relationships.
Mai Sedki, MD, MPH1, Meredith Craven, PhD1, Linda Nguyen, MD2, Irene Sonu, MD1. P2926 - A Randomized Controlled Trial of a Digital Cognitive Behavioral Therapy Application for Irritable Bowel Syndrome, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
