Monday Poster Session
Category: Endoscopy Video Forum
P2724 - Evaluating Enhanced Hemostatic Outcomes With PuraStat in Esophageal and Gastric Endoscopic Myotomy: A Case Series
- KP
Karthic Drishna Perumal, MD
Mount Carmel Health System
Powell, OH
Presenting Author(s)
1Mount Carmel Health System, Powell, OH; 2Aga Khan University Medical Center, Karachi, Sindh, Pakistan; 3The Ohio State University Wexner Medical Center, Columbus, OH
Introduction: Advances in surgical techniques have led to a shift toward minimally invasive procedures with shorter recovery times, prompting the development of endoscopic approaches for treating esophageal and gastric motility disorders, including esophageal peroral endoscopic myotomy (E-POEM) and gastric-POEM (G-POEM). Despite their benefits, complications such as mucosal trauma, postoperative pain, leaks, and delayed bleeding remain concerns. PuraStat (3-D Matrix, Tokyo, Japan), a self-assembling peptide hydrogel, has shown promise in improving clinical outcomes, but its application in gastrointestinal (GI) bleeding events during endoscopic myotomy has not been extensively studied. This study aims to evaluate its safety and efficacy in E-POEM and G-POEM procedures.
Case Description/
Methods:
We systematically reviewed patient-related clinical data, including demographics, symptoms, medical history, use of antiplatelet agents, necessity of POEM, and imaging findings in 6 patients. Procedural data included the effectiveness of endoscopic hemostasis with PuraStat, need for additional interventions, surgical field visualization, cautery usage, procedural perforation, and postprocedural bleeding outcomes. In all cases, 3 mL of PuraStat was applied as the primary hemostatic method. For E-POEM, it was sprayed over the closure site following mucosal entrance closure with endoscopic clips. In G-POEM, PuraStat was applied within the tunnel before closure.
PuraStat’s efficacy was assessed through intraprocedural and postprocedural outcomes. Intraprocedural measures included surgical field clarity, reduced electrocautery use, and bleeding requiring further intervention. Postprocedural outcomes focused on early and delayed bleeding, stratified by severity-none, mild, or severe. Secondary assessments examined symptom improvement in dysphagia using the Eckardt score and esophagram findings for E-POEM, while gastropareiss cardinal symptom index (GCSI) scores and gastric emptying results were used for G-POEM-related gastroparesis.
Discussion: To our knowledge, this study represents the first evaluation of PuraStat’s efficacy in peroral endoscopic myotomy. Our findings demonstrate 100% clinical and/or endoscopic improvement, preventing early and delayed bleeding without additional hemostatic measures, reducing cautery use, and ensuring procedural efficacy in addressing patient symptoms.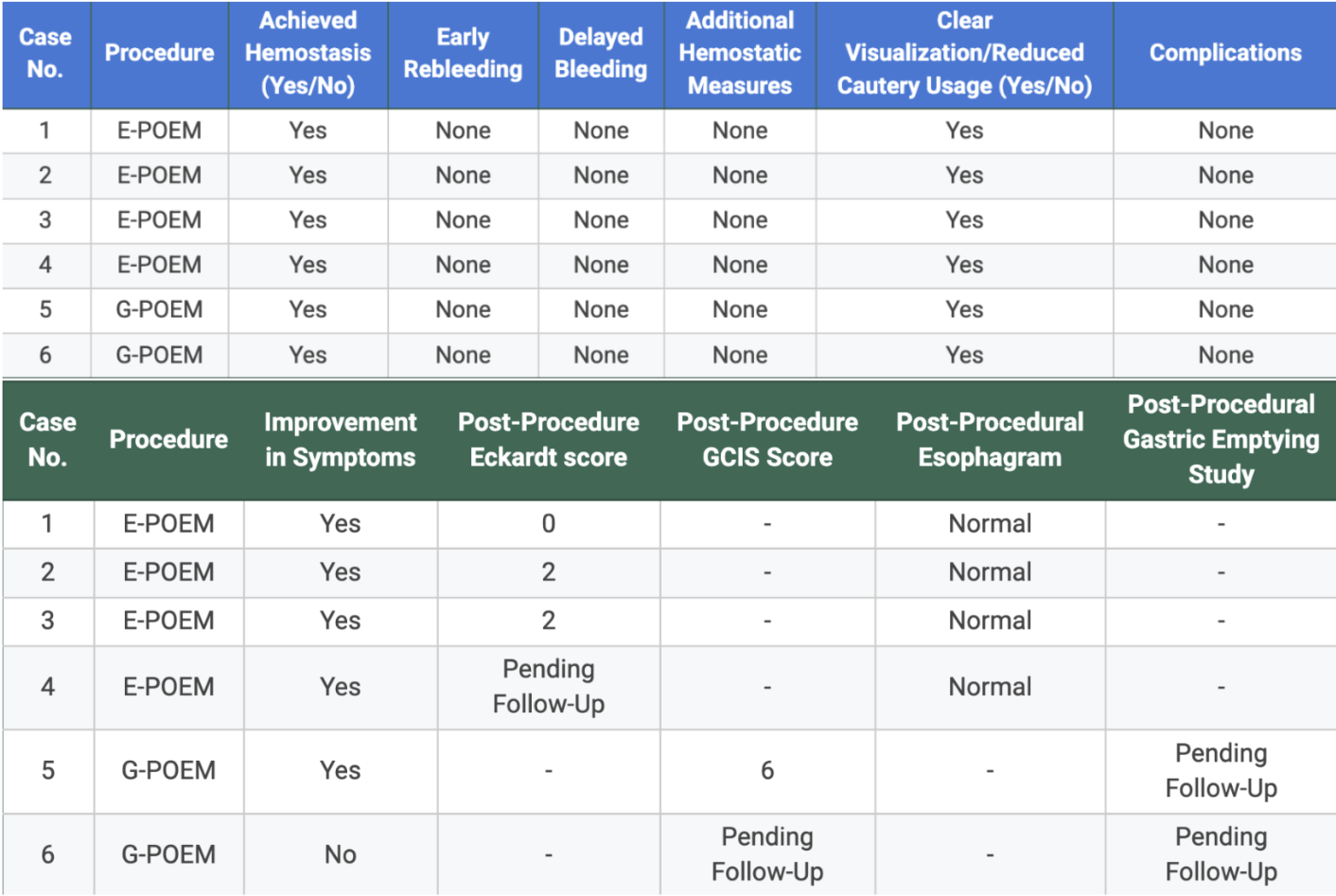
Figure: Table 1: Clinical characteristics of patients studied and indications for E-POEM and G-POEMs including age, sex, symptoms, pre-procedure Eckardt/GCIS scores, underlying comorbidities, and antithrombotic drug usage.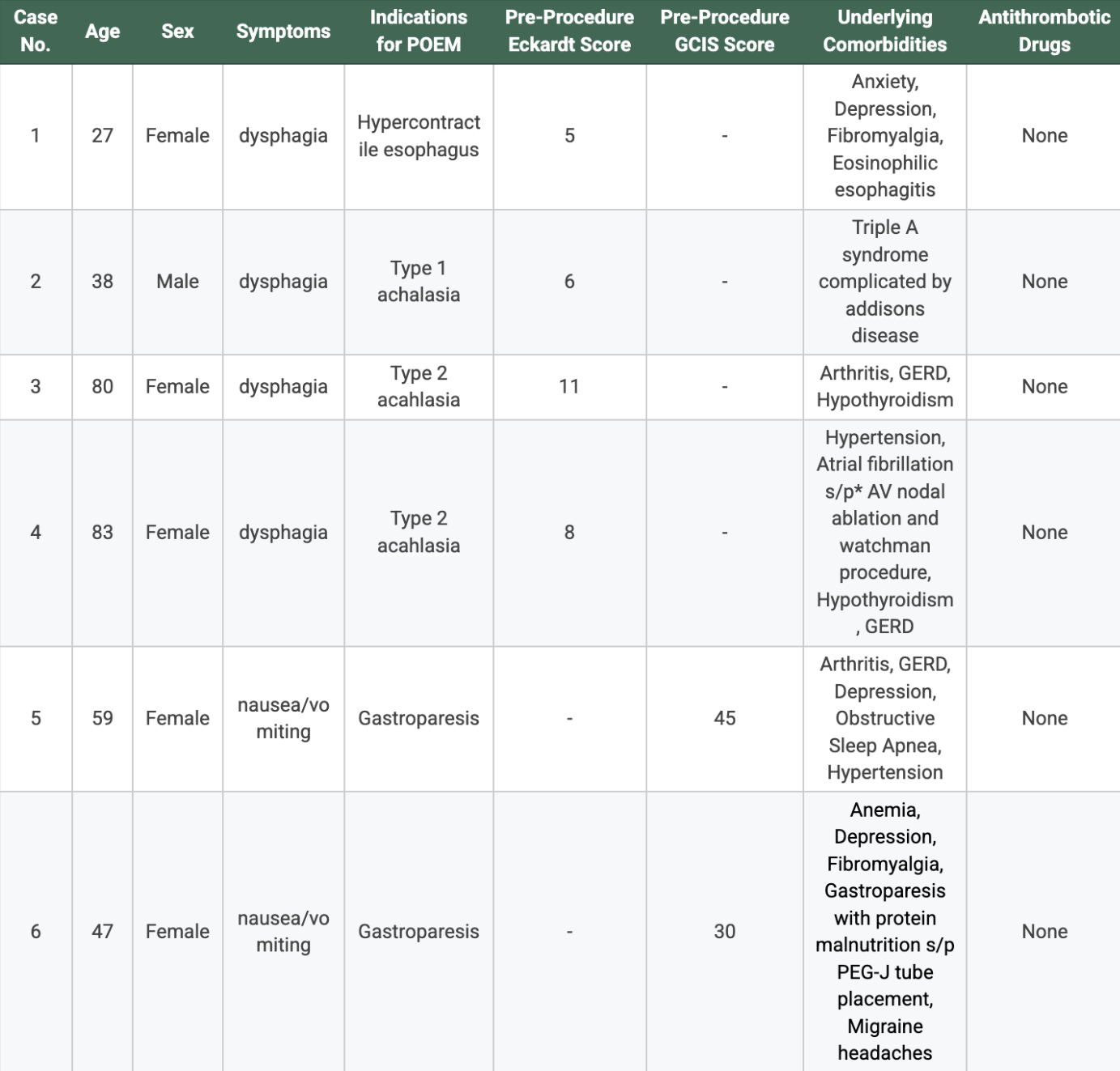
Figure: Table 2: Primary and secondary endoscopic data outcomes. The blue section displays the primary outcomes of early/delayed bleeding, additional hemostatic measures used, clear visualization/reduced cautery usage, and complications. The green section displays the secondary outcomes of improvement in symptoms, post-procedure Eckardt/GCIS scores, and post-procedure esophagram and gastric emptying study results.
Disclosures:
Karthic Drishna Perumal indicated no relevant financial relationships.
Masood Muhammad Karim indicated no relevant financial relationships.
Jordan Burlen indicated no relevant financial relationships.
Karthic Drishna Perumal, MD1, Masood Muhammad Karim, MBBS2, Jordan Burlen, MD3. P2724 - Evaluating Enhanced Hemostatic Outcomes With PuraStat in Esophageal and Gastric Endoscopic Myotomy: A Case Series, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
