Monday Poster Session
Category: Diet, Nutrition, and Obesity
P2694 - Efficacy and Safety of Tirzepatide for the Management of Obesity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials (RCTs)
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Monica Gaurdian, MBBS (she/her/hers)
university Hospitals Birmingham NHS Foundation Trust
Birmingham, India
Presenting Author(s)
Monica Gaurdian, MBBS1, Kissan Ghose, 2, Muhammad Ayyan, MBBS3, David Losier, BS4, Paulami Deshmukh, MBBS5, Maurya Patel, 6, Nadia Ahmed, MBBCh7, Mohamed Moazzam Vahora, MBBS8, Sean Ghose, BSc9, Cara Mohammad, MBBS, MHA, MSc, MRCS10, Avni Bhatia, MBBS11, Kebire Gofar, 12
1university Hospitals Birmingham NHS Foundation Trust, Birmingham, England, United Kingdom; 2University of Lethbridge, Calgary, AB, Canada; 3King Edward Medical University, Lahore, Punjab, Pakistan; 4SUNY Upstate Medical University, New York, NY; 5SMT KASHIBAI NAVALE MEDICAL COLLEGE AND GENERAL HOSPITAL, Pune, Maharashtra, India; 6Seth Gordhandas Sundardas Medical College and KEM Hospital, Mumbai, Maharashtra, India; 7Faculty of Medicine, Ain Shams University, Richmond, BC, Canada; 8King's College Hospital NHS Foundation Trust, London, England, United Kingdom; 9University of Alberta, Calgary, AB, Canada; 10SANGRE GRANDE HOSPITAL, Sangre Grande, Sangre Grande, Trinidad and Tobago; 11JAWAHARLAL NEHRU MEDICAL COLLEGE, Wardha, Maharashtra, India; 12The Ohio State University, Columbus, OH
Introduction: Obesity is a significant risk factor for Type 2 Diabetes and plays a significant role in global morbidity and mortality. While current pharmacological treatments, such as glucagon-like peptide-1 receptor agonists (GLP-1 RA), have demonstrated efficacy in weight loss and glycemic control, Tirzepatide, a dual glucose-dependent insulinotropic polypeptide, has arisen as a viable treatment option. This study aims to investigate the evidence on comparing Tirzepatide to placebo and GLP-1 RAs such as Semaglutide and Dulaglutide.
Methods: We searched electronic databases to retrieve and include all randomized controlled trials (RCTs) that analyzed the effect of tirzepatide for the management of obesity. The revised Cochrane’s “Risk of Bias" tool for randomized trials (RoB 2.0) was used to assess the risk of bias in the included studies. Using RevMan, risk ratios (RR) along with the 95% confidence intervals (95% CI) were used for dichotomous outcomes, and standard mean differences (SMD) along with the 95% confidence intervals (95% CI) were used for continuous outcomes.
Results: A total of fourteen RCTs were included in our meta-analysis, reporting data from 9968 patients. When comparing tirzepatide to placebo, our analysis showed a statistically significant difference favoring Tirzepatide dosages of 5mg, 10mg and 15mg for change in mean body weight (SMD -2.25; 95% CI: -2.62 to -1.88), weight reduction of ≥ 5% (RR 3.76; 95% CI: 3.16-4.47), weight reduction of ≥ 10% (RR 6.40; 95% CI: 5.00-8.19) and weight reduction of ≥ 15% (RR 11.32; 95% CI: 8.34-15.37). When comparing Tirzepatide vs GLP-1 agonists, our meta-analysis showed a statistically significant difference favoring Tirzepatide dosages of 5mg, 10mg and 15mg for Change in mean body weight (SMD -2.18; 95% CI: -2.70 to -1.66), weight reduction of ≥ 5% (RR 2.96; 95% CI: 2.14-4.10), weight reduction of ≥ 10% (RR 3.25; 95% CI: 2.28-4.64), and weight reduction of ≥ 15% (RR 4.72; 95% CI: 2.75, 8.11).
Discussion: This meta-analysis confirms that tirzepatide is an effective pharmacologic option for obesity treatment, demonstrating statistically significant reductions in mean body weight and BMI. Given its recent FDA approval for obesity, tirzepatide represents a promising advancement in obesity treatment, though further long-term studies are needed to assess sustained efficacy and long-term adverse effects.
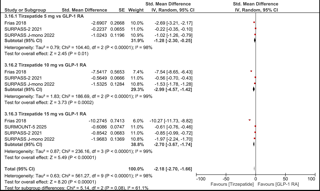
Figure: Comparison of change in body weight (standardized mean difference) between patients receiving Tirzepatide or GLP-1 RA.. IV, inverse variance.
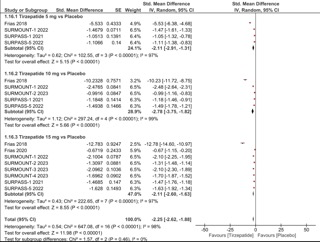
Figure: Comparison of change in body weight (standardized mean difference) between patients receiving Tirzepatide or placebo.. IV, inverse variance.
Disclosures:
Monica Gaurdian indicated no relevant financial relationships.
Kissan Ghose indicated no relevant financial relationships.
Muhammad Ayyan indicated no relevant financial relationships.
David Losier indicated no relevant financial relationships.
Paulami Deshmukh indicated no relevant financial relationships.
Maurya Patel indicated no relevant financial relationships.
Nadia Ahmed indicated no relevant financial relationships.
Mohamed Moazzam Vahora indicated no relevant financial relationships.
Sean Ghose indicated no relevant financial relationships.
Cara Mohammad indicated no relevant financial relationships.
Avni Bhatia indicated no relevant financial relationships.
Kebire Gofar indicated no relevant financial relationships.
Monica Gaurdian, MBBS1, Kissan Ghose, 2, Muhammad Ayyan, MBBS3, David Losier, BS4, Paulami Deshmukh, MBBS5, Maurya Patel, 6, Nadia Ahmed, MBBCh7, Mohamed Moazzam Vahora, MBBS8, Sean Ghose, BSc9, Cara Mohammad, MBBS, MHA, MSc, MRCS10, Avni Bhatia, MBBS11, Kebire Gofar, 12. P2694 - Efficacy and Safety of Tirzepatide for the Management of Obesity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials (RCTs), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1university Hospitals Birmingham NHS Foundation Trust, Birmingham, England, United Kingdom; 2University of Lethbridge, Calgary, AB, Canada; 3King Edward Medical University, Lahore, Punjab, Pakistan; 4SUNY Upstate Medical University, New York, NY; 5SMT KASHIBAI NAVALE MEDICAL COLLEGE AND GENERAL HOSPITAL, Pune, Maharashtra, India; 6Seth Gordhandas Sundardas Medical College and KEM Hospital, Mumbai, Maharashtra, India; 7Faculty of Medicine, Ain Shams University, Richmond, BC, Canada; 8King's College Hospital NHS Foundation Trust, London, England, United Kingdom; 9University of Alberta, Calgary, AB, Canada; 10SANGRE GRANDE HOSPITAL, Sangre Grande, Sangre Grande, Trinidad and Tobago; 11JAWAHARLAL NEHRU MEDICAL COLLEGE, Wardha, Maharashtra, India; 12The Ohio State University, Columbus, OH
Introduction: Obesity is a significant risk factor for Type 2 Diabetes and plays a significant role in global morbidity and mortality. While current pharmacological treatments, such as glucagon-like peptide-1 receptor agonists (GLP-1 RA), have demonstrated efficacy in weight loss and glycemic control, Tirzepatide, a dual glucose-dependent insulinotropic polypeptide, has arisen as a viable treatment option. This study aims to investigate the evidence on comparing Tirzepatide to placebo and GLP-1 RAs such as Semaglutide and Dulaglutide.
Methods: We searched electronic databases to retrieve and include all randomized controlled trials (RCTs) that analyzed the effect of tirzepatide for the management of obesity. The revised Cochrane’s “Risk of Bias" tool for randomized trials (RoB 2.0) was used to assess the risk of bias in the included studies. Using RevMan, risk ratios (RR) along with the 95% confidence intervals (95% CI) were used for dichotomous outcomes, and standard mean differences (SMD) along with the 95% confidence intervals (95% CI) were used for continuous outcomes.
Results: A total of fourteen RCTs were included in our meta-analysis, reporting data from 9968 patients. When comparing tirzepatide to placebo, our analysis showed a statistically significant difference favoring Tirzepatide dosages of 5mg, 10mg and 15mg for change in mean body weight (SMD -2.25; 95% CI: -2.62 to -1.88), weight reduction of ≥ 5% (RR 3.76; 95% CI: 3.16-4.47), weight reduction of ≥ 10% (RR 6.40; 95% CI: 5.00-8.19) and weight reduction of ≥ 15% (RR 11.32; 95% CI: 8.34-15.37). When comparing Tirzepatide vs GLP-1 agonists, our meta-analysis showed a statistically significant difference favoring Tirzepatide dosages of 5mg, 10mg and 15mg for Change in mean body weight (SMD -2.18; 95% CI: -2.70 to -1.66), weight reduction of ≥ 5% (RR 2.96; 95% CI: 2.14-4.10), weight reduction of ≥ 10% (RR 3.25; 95% CI: 2.28-4.64), and weight reduction of ≥ 15% (RR 4.72; 95% CI: 2.75, 8.11).
Discussion: This meta-analysis confirms that tirzepatide is an effective pharmacologic option for obesity treatment, demonstrating statistically significant reductions in mean body weight and BMI. Given its recent FDA approval for obesity, tirzepatide represents a promising advancement in obesity treatment, though further long-term studies are needed to assess sustained efficacy and long-term adverse effects.

Figure: Comparison of change in body weight (standardized mean difference) between patients receiving Tirzepatide or GLP-1 RA.. IV, inverse variance.

Figure: Comparison of change in body weight (standardized mean difference) between patients receiving Tirzepatide or placebo.. IV, inverse variance.
Disclosures:
Monica Gaurdian indicated no relevant financial relationships.
Kissan Ghose indicated no relevant financial relationships.
Muhammad Ayyan indicated no relevant financial relationships.
David Losier indicated no relevant financial relationships.
Paulami Deshmukh indicated no relevant financial relationships.
Maurya Patel indicated no relevant financial relationships.
Nadia Ahmed indicated no relevant financial relationships.
Mohamed Moazzam Vahora indicated no relevant financial relationships.
Sean Ghose indicated no relevant financial relationships.
Cara Mohammad indicated no relevant financial relationships.
Avni Bhatia indicated no relevant financial relationships.
Kebire Gofar indicated no relevant financial relationships.
Monica Gaurdian, MBBS1, Kissan Ghose, 2, Muhammad Ayyan, MBBS3, David Losier, BS4, Paulami Deshmukh, MBBS5, Maurya Patel, 6, Nadia Ahmed, MBBCh7, Mohamed Moazzam Vahora, MBBS8, Sean Ghose, BSc9, Cara Mohammad, MBBS, MHA, MSc, MRCS10, Avni Bhatia, MBBS11, Kebire Gofar, 12. P2694 - Efficacy and Safety of Tirzepatide for the Management of Obesity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials (RCTs), ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
