Monday Poster Session
Category: Diet, Nutrition, and Obesity
P2686 - Building an Endoscopic Bariatric Program in an FDA-Approved Era: 1-Year Interim Analysis of Insurance Coverage and Clinical Outcomes
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- TM
Thomas R. McCarty, MD, MPH
Houston Methodist Hospital
Houston, TX
Presenting Author(s)
Award: ACG Presidential Poster Award
Ronan Allencherril, MD, Thomas R. McCarty, MD, MPH
Houston Methodist Hospital, Houston, TX
Introduction: While the first endoscopic suturing system was FDA approved for class I-III obesity in June 2022, there remains no real-world data regarding insurance coverage for endoscopic sleeve gastroplasty (ESG) or transoral outlet reduction (TORe). The primary aim of this study was to assess insurance coverage and the efficacy and safety of ESG and TORe.
Methods: This was a retrospective study of a newly established multi-disciplinary endoscopic bariatric program at a tertiary care hospital. The program was established September 2023 with data collected through May 2025. All patients underwent ESG or TORe with an endoscopic suture-based system specifically approved for the treatment of obesity. All patients were evaluated by a registered dietitian and behavioral psychologist with insurance pre-authorization using CPT 43999 submitted prior to undergoing the procedure. The primary outcome was pre-authorization success via insurance. Secondary outcomes included percent total body weight loss (%TWL) 12 months after ESG/TORe. Additional outcomes included improvement in obesity-associated comorbid conditions.
Results: A total of 35 patients were evaluated with plan for ESG and TORe during the first 15 months the program was established. A mix of payer systems were included with 52.94% of ESG patients and 20.00% of TORe patients receiving an initial denial. Appeal and/or peer-to-peer was successfully overturned for 33.33% of ESG patients and 0.00% of TORe patients. Of these initial 35 patients, 80.00% (n=28) of patients eventually underwent ESG and TORe. Baseline patient, payer, and outcomes characteristics are summarized in Table 1. Mean age of patients was 50.46 (11.20) years with 85.71% female. Twenty-eight percent of patients were on concomitant pharmacotherapy. The TWL for all procedures was 18.60% and 21.84% at 6 months and 12 months, respectively. No serious adverse events occurred with the one patient readmitted in the TORe group within 90-days for gastrojejunostomy stenosis. Comorbidity improvement was seen in at least 50% of patients in both ESG and TORe groups.
Discussion: Both ESG and TORe were associated with significant weight loss and no serious adverse events. Yet, despite ESG and TORe achieving 12-month TWL of 16.45% and 25.16%, respectively, only half of ESG patients and one-fifth of TORe patients received initial insurance authorization for these endoscopic FDA approved procedures. Improved coverage is required to increase adoption of endoscopic bariatric procedures.
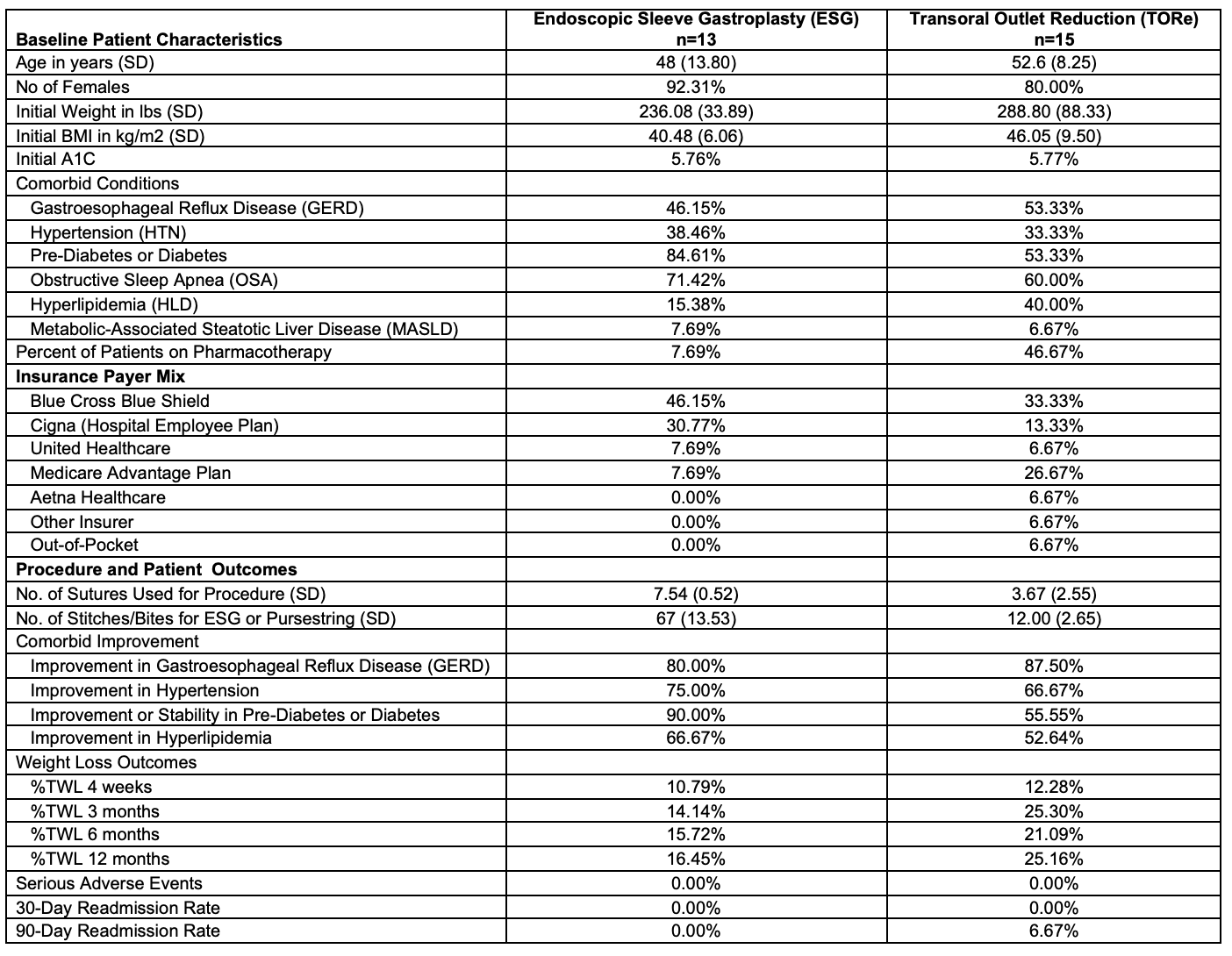
Figure: Table 1. Baseline Characteristics and Clinical Outcomes for ESG and TORe
Disclosures:
Ronan Allencherril indicated no relevant financial relationships.
Thomas McCarty: Covidien/Medtronic – Consultant. Endoquest Robotics – Consultant.
Ronan Allencherril, MD, Thomas R. McCarty, MD, MPH. P2686 - Building an Endoscopic Bariatric Program in an FDA-Approved Era: 1-Year Interim Analysis of Insurance Coverage and Clinical Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Ronan Allencherril, MD, Thomas R. McCarty, MD, MPH
Houston Methodist Hospital, Houston, TX
Introduction: While the first endoscopic suturing system was FDA approved for class I-III obesity in June 2022, there remains no real-world data regarding insurance coverage for endoscopic sleeve gastroplasty (ESG) or transoral outlet reduction (TORe). The primary aim of this study was to assess insurance coverage and the efficacy and safety of ESG and TORe.
Methods: This was a retrospective study of a newly established multi-disciplinary endoscopic bariatric program at a tertiary care hospital. The program was established September 2023 with data collected through May 2025. All patients underwent ESG or TORe with an endoscopic suture-based system specifically approved for the treatment of obesity. All patients were evaluated by a registered dietitian and behavioral psychologist with insurance pre-authorization using CPT 43999 submitted prior to undergoing the procedure. The primary outcome was pre-authorization success via insurance. Secondary outcomes included percent total body weight loss (%TWL) 12 months after ESG/TORe. Additional outcomes included improvement in obesity-associated comorbid conditions.
Results: A total of 35 patients were evaluated with plan for ESG and TORe during the first 15 months the program was established. A mix of payer systems were included with 52.94% of ESG patients and 20.00% of TORe patients receiving an initial denial. Appeal and/or peer-to-peer was successfully overturned for 33.33% of ESG patients and 0.00% of TORe patients. Of these initial 35 patients, 80.00% (n=28) of patients eventually underwent ESG and TORe. Baseline patient, payer, and outcomes characteristics are summarized in Table 1. Mean age of patients was 50.46 (11.20) years with 85.71% female. Twenty-eight percent of patients were on concomitant pharmacotherapy. The TWL for all procedures was 18.60% and 21.84% at 6 months and 12 months, respectively. No serious adverse events occurred with the one patient readmitted in the TORe group within 90-days for gastrojejunostomy stenosis. Comorbidity improvement was seen in at least 50% of patients in both ESG and TORe groups.
Discussion: Both ESG and TORe were associated with significant weight loss and no serious adverse events. Yet, despite ESG and TORe achieving 12-month TWL of 16.45% and 25.16%, respectively, only half of ESG patients and one-fifth of TORe patients received initial insurance authorization for these endoscopic FDA approved procedures. Improved coverage is required to increase adoption of endoscopic bariatric procedures.

Figure: Table 1. Baseline Characteristics and Clinical Outcomes for ESG and TORe
Disclosures:
Ronan Allencherril indicated no relevant financial relationships.
Thomas McCarty: Covidien/Medtronic – Consultant. Endoquest Robotics – Consultant.
Ronan Allencherril, MD, Thomas R. McCarty, MD, MPH. P2686 - Building an Endoscopic Bariatric Program in an FDA-Approved Era: 1-Year Interim Analysis of Insurance Coverage and Clinical Outcomes, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

