Monday Poster Session
Category: Colorectal Cancer Prevention
P2631 - One-Time Colorectal Cancer Screening with Next-Generation mt-sDNA vs FIT: Clinical and Economic Impact Using a Comprehensive Cost-Calculator Economic Model
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Derek Ebner, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
A. Mark Fendrick, MD1, Derek Ebner, MD2, Michael Dore, MD3, Mohammad Dehghani, PhD4
1University of Michigan, Ann Arbor, MI; 2Mayo Clinic, Rochester, MN; 3Duke University School of Medicine, Durham, NC; 4Northeastern University, Boston, MA
Introduction: Colorectal cancer (CRC) remains one of the leading causes of cancer-related morbidity and mortality in the US. Multiple stool-based CRC screening strategies with varying intervals are currently recommended by the United States Preventive Services Task Force. With the recent introduction of triennial screening with the next-generation multi-target stool DNA (ng mt-sDNA) test, it is timely to explore its estimated impact compared to annual screening with the fecal immunochemical test (FIT), using predicted real-world adherence and performance characteristics from clinical trials. Many prior analyses assumed perfect adherence to both screening and follow-up colonoscopy, which can lead to biased results.
Methods: A cohort-based model was developed with prevalence of advanced precancerous lesions (APL) and CRC obtained from published clinical trials. A hypothetical cohort of 1 million average-risk individuals was modeled to simulate the estimated outcomes from one round of ng mt-sDNA versus FIT screening. Test performance was based on the BLUE-C clinical trial with ng mt-sDNA compared to FIT sensitivity for CRC and APL. Based on published literature, real-world adherence was modeled at 71.3% for ng mt-sDNA and 31.8% for FIT completion, and 72.1% and 46.0%, for follow-up colonoscopy. Screening and treatment costs were included based on reported literature. Outcomes included the number of detected CRC and APL cases, CRC cases averted through APL detection, and associated costs for each screening strategy.
Results: The model predicted significantly more patients screened with ng mt-sDNA compared to FIT (713,000 vs 318,000), with 5.7-fold higher achievement of HEDIS® quality targets over 3 years. ng mt-sDNA detected 5.5 times more APL (21,701 vs 3,328) and 3.7 times more CRC cases (2,090 vs 440) than FIT screening. CRC cases averted were 1,736 with ng mt-sDNA, 5.5 times higher than FIT (266 cases). Finally, ng mt-sDNA reduced CRC treatment costs by 3% and total (screening and treatment) costs by 2% ($1,388 M vs $1,423 M) compared to FIT.
Discussion: This analysis of one-time screening suggests that ng mt-sDNA can more effectively detect and avert CRC cases and related costs as compared to FIT, due to differences in performance, completion, and follow up colonoscopy rates. Expanded use of ng mt-sDNA CRC screening tests represents a practical, scalable strategy to improve clinical and economic outcomes achieved through noninvasive CRC screening.
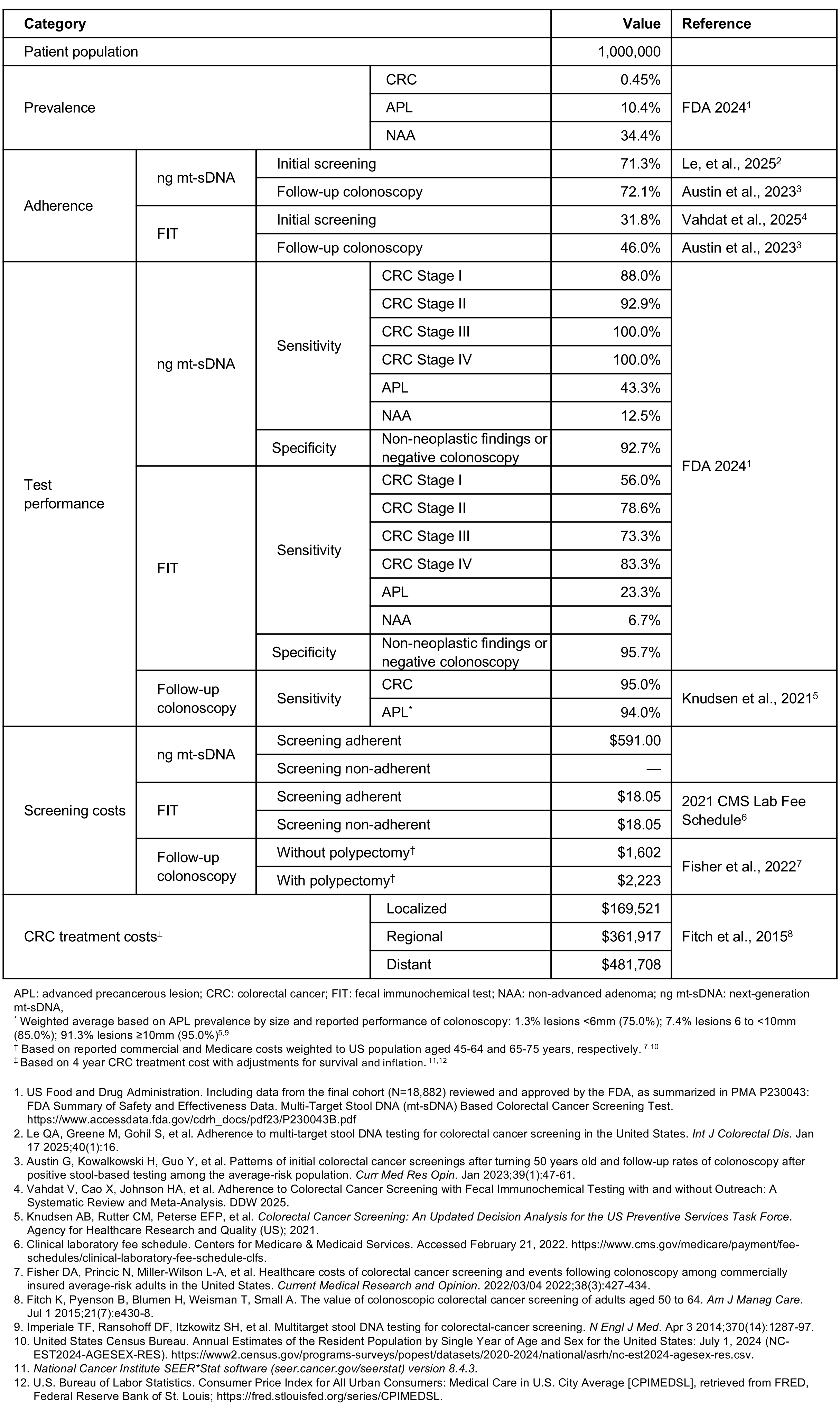
Figure: Table. Inputs for the economic modeling analysis

Figure: Figure. Clinical (panel A), performance measure (panel B), and economic (panel C) impact of one-time screening with ng-mt-sDNA vs FIT (results are per 1 M screening eligible population. Abbreviations: APL: Advanced Precancerous Lesions; CRC: Colorectal Cancer; FIT: Fecal Immunochemical Testing; HEDIS®: Healthcare Effectiveness Data and Information Set; ng mt-sDNA: Next Generation multi target stool DNA)
Disclosures:
A. Mark Fendrick: AbbVie – Consultant. Agency for Healthcare Research and Quality – Grant/Research Support. Amgen – Consultant. Arnold Ventures – Grant/Research Support. Centers for Medicare and Medicaid Services. – Grant/Research Support. Centivo – Consultant. Community Oncology Association – Consultant. Covered California – Consultant. EmblemHealth – Consultant. Exact Sciences – Consultant. Freedman Health – Consultant. Gary and Mary West Health Policy Center – Grant/Research Support. GRAIL – Consultant. Harvard University – Consultant. Health & Wellness Innovations – Consultant. Health at Scale Technologies – Consultant. MedZed – Consultant. National Pharmaceutical Council – Grant/Research Support. Patient-Centered Outcomes Research Institute – Grant/Research Support. Penguin Pay – Consultant. Pharmaceutical Research and Manufacturers of America – Grant/Research Support. Risalto – Consultant. Robert Wood Johnson Foundation – Grant/Research Support. Sempre Health – Consultant. State of Michigan – Grant/Research Support. State of Minnesota – Consultant. U.S. Department of Defense – Consultant. Virginia Center for Health Innovation – Consultant. Wellthy – Consultant. Zansors – Consultant.
Derek Ebner: Exact Sciences – Consultant.
Michael Dore indicated no relevant financial relationships.
Mohammad Dehghani: Exact Sciences – Advisor or Review Panel Member, Consultant. Exact Sciences – Consultant.
A. Mark Fendrick, MD1, Derek Ebner, MD2, Michael Dore, MD3, Mohammad Dehghani, PhD4. P2631 - One-Time Colorectal Cancer Screening with Next-Generation mt-sDNA vs FIT: Clinical and Economic Impact Using a Comprehensive Cost-Calculator Economic Model, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Michigan, Ann Arbor, MI; 2Mayo Clinic, Rochester, MN; 3Duke University School of Medicine, Durham, NC; 4Northeastern University, Boston, MA
Introduction: Colorectal cancer (CRC) remains one of the leading causes of cancer-related morbidity and mortality in the US. Multiple stool-based CRC screening strategies with varying intervals are currently recommended by the United States Preventive Services Task Force. With the recent introduction of triennial screening with the next-generation multi-target stool DNA (ng mt-sDNA) test, it is timely to explore its estimated impact compared to annual screening with the fecal immunochemical test (FIT), using predicted real-world adherence and performance characteristics from clinical trials. Many prior analyses assumed perfect adherence to both screening and follow-up colonoscopy, which can lead to biased results.
Methods: A cohort-based model was developed with prevalence of advanced precancerous lesions (APL) and CRC obtained from published clinical trials. A hypothetical cohort of 1 million average-risk individuals was modeled to simulate the estimated outcomes from one round of ng mt-sDNA versus FIT screening. Test performance was based on the BLUE-C clinical trial with ng mt-sDNA compared to FIT sensitivity for CRC and APL. Based on published literature, real-world adherence was modeled at 71.3% for ng mt-sDNA and 31.8% for FIT completion, and 72.1% and 46.0%, for follow-up colonoscopy. Screening and treatment costs were included based on reported literature. Outcomes included the number of detected CRC and APL cases, CRC cases averted through APL detection, and associated costs for each screening strategy.
Results: The model predicted significantly more patients screened with ng mt-sDNA compared to FIT (713,000 vs 318,000), with 5.7-fold higher achievement of HEDIS® quality targets over 3 years. ng mt-sDNA detected 5.5 times more APL (21,701 vs 3,328) and 3.7 times more CRC cases (2,090 vs 440) than FIT screening. CRC cases averted were 1,736 with ng mt-sDNA, 5.5 times higher than FIT (266 cases). Finally, ng mt-sDNA reduced CRC treatment costs by 3% and total (screening and treatment) costs by 2% ($1,388 M vs $1,423 M) compared to FIT.
Discussion: This analysis of one-time screening suggests that ng mt-sDNA can more effectively detect and avert CRC cases and related costs as compared to FIT, due to differences in performance, completion, and follow up colonoscopy rates. Expanded use of ng mt-sDNA CRC screening tests represents a practical, scalable strategy to improve clinical and economic outcomes achieved through noninvasive CRC screening.

Figure: Table. Inputs for the economic modeling analysis

Figure: Figure. Clinical (panel A), performance measure (panel B), and economic (panel C) impact of one-time screening with ng-mt-sDNA vs FIT (results are per 1 M screening eligible population. Abbreviations: APL: Advanced Precancerous Lesions; CRC: Colorectal Cancer; FIT: Fecal Immunochemical Testing; HEDIS®: Healthcare Effectiveness Data and Information Set; ng mt-sDNA: Next Generation multi target stool DNA)
Disclosures:
A. Mark Fendrick: AbbVie – Consultant. Agency for Healthcare Research and Quality – Grant/Research Support. Amgen – Consultant. Arnold Ventures – Grant/Research Support. Centers for Medicare and Medicaid Services. – Grant/Research Support. Centivo – Consultant. Community Oncology Association – Consultant. Covered California – Consultant. EmblemHealth – Consultant. Exact Sciences – Consultant. Freedman Health – Consultant. Gary and Mary West Health Policy Center – Grant/Research Support. GRAIL – Consultant. Harvard University – Consultant. Health & Wellness Innovations – Consultant. Health at Scale Technologies – Consultant. MedZed – Consultant. National Pharmaceutical Council – Grant/Research Support. Patient-Centered Outcomes Research Institute – Grant/Research Support. Penguin Pay – Consultant. Pharmaceutical Research and Manufacturers of America – Grant/Research Support. Risalto – Consultant. Robert Wood Johnson Foundation – Grant/Research Support. Sempre Health – Consultant. State of Michigan – Grant/Research Support. State of Minnesota – Consultant. U.S. Department of Defense – Consultant. Virginia Center for Health Innovation – Consultant. Wellthy – Consultant. Zansors – Consultant.
Derek Ebner: Exact Sciences – Consultant.
Michael Dore indicated no relevant financial relationships.
Mohammad Dehghani: Exact Sciences – Advisor or Review Panel Member, Consultant. Exact Sciences – Consultant.
A. Mark Fendrick, MD1, Derek Ebner, MD2, Michael Dore, MD3, Mohammad Dehghani, PhD4. P2631 - One-Time Colorectal Cancer Screening with Next-Generation mt-sDNA vs FIT: Clinical and Economic Impact Using a Comprehensive Cost-Calculator Economic Model, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

