Monday Poster Session
Category: Colon
P2595 - Zolbetuximab-Induced Colitis Leading to Colonic Perforation: A Call for Researching a Novel Drug
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- ZB
Zeina Bani Hani, MBBS
Department of Medicine, George Washington University School of Medicine and Health Sciences
Washington, DC
Presenting Author(s)
Zeina Bani Hani, MBBS1, Romy Chamoun, MD2, Simran Gupta, MD3, Robert S.. Gordon, DO, MS4, Jennie Zhang, DO5, Huimin Yu, MD, PhD6, Marie L. Borum, MD, EdD, MPH, FACG3
1Department of Medicine, George Washington University School of Medicine and Health Sciences, Washington, DC; 2George Washington University School of Medicine and Health Sciences, Washington, DC; 3Division of Gastroenterology and Liver Disease, Department of Medicine, George Washington University School of Medicine and Health Sciences, Washington, DC; 4George Washington University School of Medicine and Health Sciences, Arlington, WA; 5George Washington University, Washington, DC; 6George Washington University Hospital, Washington, DC
Introduction: Zolbetuximab is a novel chimeric monoclonal antibody that is used in patients with advanced gastric or
gastro-esophageal junction (G/GEJ) adenocarcinoma. It targets Claudin 18.2 is a protein that is often
overexpressed in gastric and GEJ cancers. Acute diarrhea is a recognized adverse reaction associated
with Zolbetuximab. We present the first documented case of a female with poorly differentiated gastric
adenocarcinoma that developed acute colitis complicated by perforation after initiation of
Zolbetuximab.
Case Description/
Methods: An 85-year-old female with history of hypertension, atrial fibrillation, mitral regurgitation, congenital
hypertriglyceridemia, osteoporosis, and moderately to poorly differentiated adenocarcinoma of the
stomach diagnosed 4 months prior to admission. She was on standard chemotherapy “FOLFOX” and
developed acute profuse watery diarrhea after addition of Zolbetuximab. She reported 12-15 bowel
movements daily after starting Zolbetuximab. Stool studies for gastrointestinal infections were negative.
Abdominal CT was notable for contained rectal wall perforation, pneumatosis intestinalis in the
transverse colon, and hyperemic appearance of small-bowel loops, concerning for enteritis. The diarrhea
persisted through scheduled Lomotil, Loperamide, and cholestyramine. A repeat abdominal CT
demonstrating spontaneous resolution of the rectal perforation and interval decrease of the transverse
colon pneumatosis. Sigmoidoscopy with water immersion was notable for an ulcer located in the
transverse colon and severe localized inflammation of the sigmoid colon and diverticulosis with biopsy
demonstrating acute inflammation, negative for CMV. Her symptoms gradually improved with
conservative management.
Discussion: The most common adverse reactions in patients on zolbetuximab with chemotherapy are nausea,
vomiting, fatigue, and diarrhea. Management of Zolbetuximab-induced acute diarrhea involves
supportive care, including assessment of severity, correction of fluid and electrolyte imbalances, and
antidiarrheal agents as clinically indicated. Given the onset of symptoms shortly after initiation of
zolbetuximab without evidence of infection, this case represents zolbetuximab-induced colitis
complicated with colonic perforation which is not a well-documented adverse reaction. This is a
potentially serious complication to a novel agent which may require further evaluation in phase IV
clinical trials and monitoring in clinical practice.
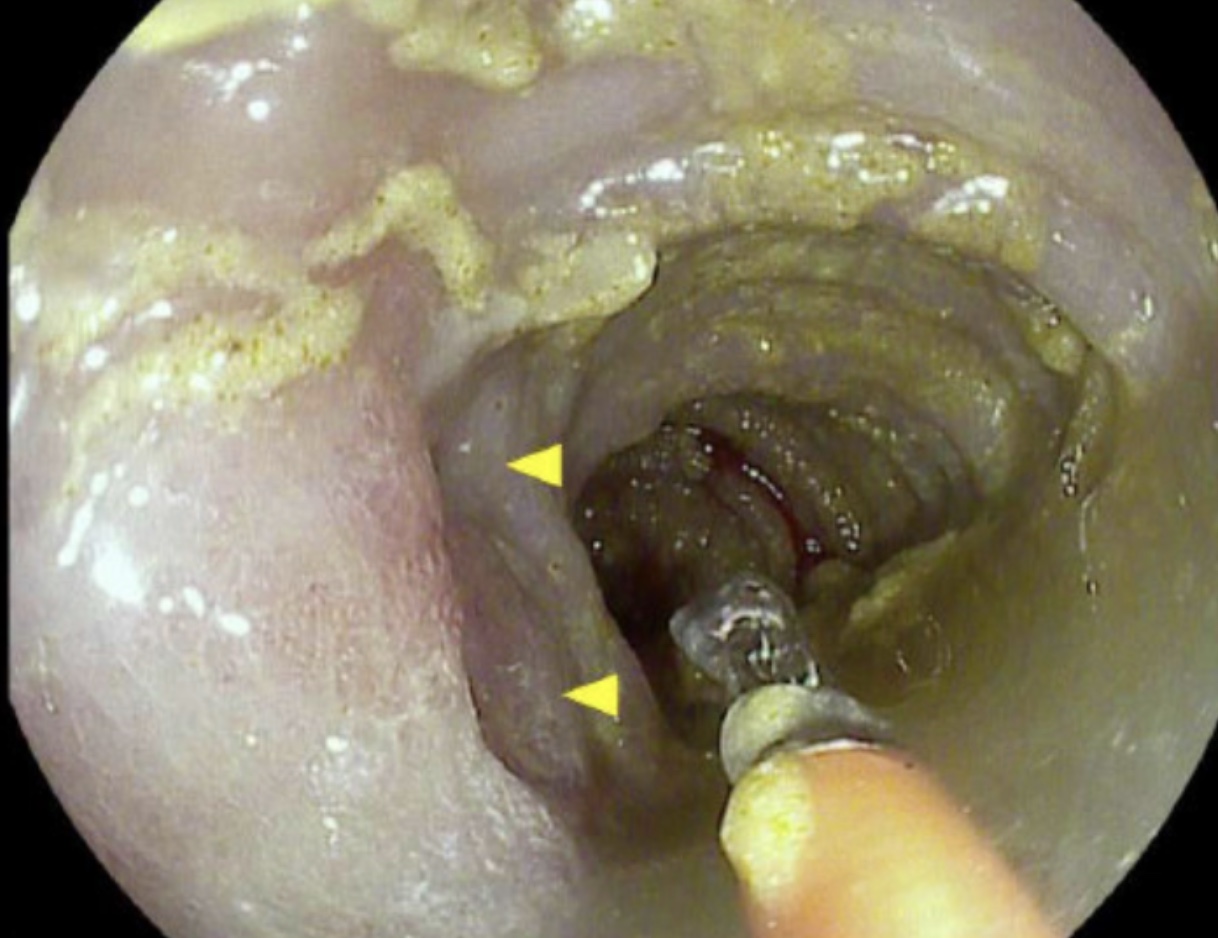
Figure: There is discontinuity of the left lateral rectal wall with surrounding free air
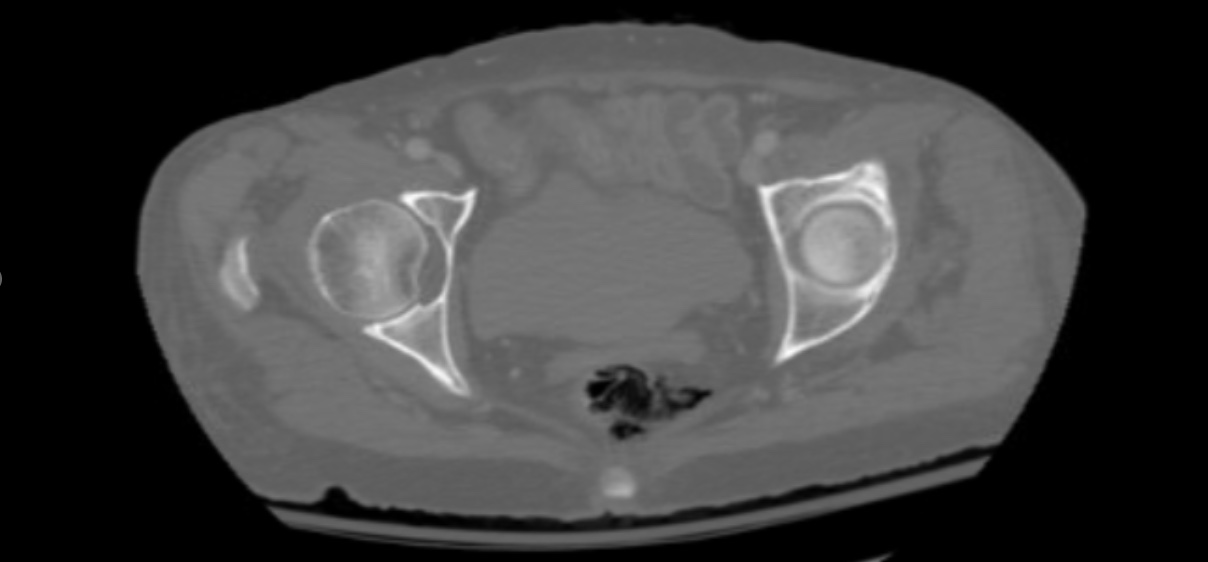
Figure: Flexible Sigmoidoscopy: Sigmoid Colon demonstrating localized inflammation
Disclosures:
Zeina Bani Hani indicated no relevant financial relationships.
Romy Chamoun indicated no relevant financial relationships.
Simran Gupta indicated no relevant financial relationships.
Robert Gordon indicated no relevant financial relationships.
Jennie Zhang indicated no relevant financial relationships.
Huimin Yu indicated no relevant financial relationships.
Marie Borum indicated no relevant financial relationships.
Zeina Bani Hani, MBBS1, Romy Chamoun, MD2, Simran Gupta, MD3, Robert S.. Gordon, DO, MS4, Jennie Zhang, DO5, Huimin Yu, MD, PhD6, Marie L. Borum, MD, EdD, MPH, FACG3. P2595 - Zolbetuximab-Induced Colitis Leading to Colonic Perforation: A Call for Researching a Novel Drug, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Department of Medicine, George Washington University School of Medicine and Health Sciences, Washington, DC; 2George Washington University School of Medicine and Health Sciences, Washington, DC; 3Division of Gastroenterology and Liver Disease, Department of Medicine, George Washington University School of Medicine and Health Sciences, Washington, DC; 4George Washington University School of Medicine and Health Sciences, Arlington, WA; 5George Washington University, Washington, DC; 6George Washington University Hospital, Washington, DC
Introduction: Zolbetuximab is a novel chimeric monoclonal antibody that is used in patients with advanced gastric or
gastro-esophageal junction (G/GEJ) adenocarcinoma. It targets Claudin 18.2 is a protein that is often
overexpressed in gastric and GEJ cancers. Acute diarrhea is a recognized adverse reaction associated
with Zolbetuximab. We present the first documented case of a female with poorly differentiated gastric
adenocarcinoma that developed acute colitis complicated by perforation after initiation of
Zolbetuximab.
Case Description/
Methods: An 85-year-old female with history of hypertension, atrial fibrillation, mitral regurgitation, congenital
hypertriglyceridemia, osteoporosis, and moderately to poorly differentiated adenocarcinoma of the
stomach diagnosed 4 months prior to admission. She was on standard chemotherapy “FOLFOX” and
developed acute profuse watery diarrhea after addition of Zolbetuximab. She reported 12-15 bowel
movements daily after starting Zolbetuximab. Stool studies for gastrointestinal infections were negative.
Abdominal CT was notable for contained rectal wall perforation, pneumatosis intestinalis in the
transverse colon, and hyperemic appearance of small-bowel loops, concerning for enteritis. The diarrhea
persisted through scheduled Lomotil, Loperamide, and cholestyramine. A repeat abdominal CT
demonstrating spontaneous resolution of the rectal perforation and interval decrease of the transverse
colon pneumatosis. Sigmoidoscopy with water immersion was notable for an ulcer located in the
transverse colon and severe localized inflammation of the sigmoid colon and diverticulosis with biopsy
demonstrating acute inflammation, negative for CMV. Her symptoms gradually improved with
conservative management.
Discussion: The most common adverse reactions in patients on zolbetuximab with chemotherapy are nausea,
vomiting, fatigue, and diarrhea. Management of Zolbetuximab-induced acute diarrhea involves
supportive care, including assessment of severity, correction of fluid and electrolyte imbalances, and
antidiarrheal agents as clinically indicated. Given the onset of symptoms shortly after initiation of
zolbetuximab without evidence of infection, this case represents zolbetuximab-induced colitis
complicated with colonic perforation which is not a well-documented adverse reaction. This is a
potentially serious complication to a novel agent which may require further evaluation in phase IV
clinical trials and monitoring in clinical practice.

Figure: There is discontinuity of the left lateral rectal wall with surrounding free air

Figure: Flexible Sigmoidoscopy: Sigmoid Colon demonstrating localized inflammation
Disclosures:
Zeina Bani Hani indicated no relevant financial relationships.
Romy Chamoun indicated no relevant financial relationships.
Simran Gupta indicated no relevant financial relationships.
Robert Gordon indicated no relevant financial relationships.
Jennie Zhang indicated no relevant financial relationships.
Huimin Yu indicated no relevant financial relationships.
Marie Borum indicated no relevant financial relationships.
Zeina Bani Hani, MBBS1, Romy Chamoun, MD2, Simran Gupta, MD3, Robert S.. Gordon, DO, MS4, Jennie Zhang, DO5, Huimin Yu, MD, PhD6, Marie L. Borum, MD, EdD, MPH, FACG3. P2595 - Zolbetuximab-Induced Colitis Leading to Colonic Perforation: A Call for Researching a Novel Drug, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
