Tuesday Poster Session
Category: Colon
P4626 - Vibrating Capsules: Revolutionizing Constipation Management or Just a Shaky Gimmick?

Charmi Patel, MD (she/her/hers)
Rutgers New Jersey Medical School
Newark, NJ
Presenting Author(s)
Rutgers New Jersey Medical School, Newark, NJ
Introduction:
Vibrating capsules are orally ingested, programmable devices that vibrate within the colonic tract to stimulate bowel movements. It is a non-drug alternative for individuals suffering from chronic constipation. The capsule requires an activation pod and a mobile app which triggers vibration. Approved by the FDA in 2023, this innovative approach provides new hope for patients with chronic constipation who have not responded to multiple pharmacological or over-the-counter treatments.
Case Description/
Methods:
A 47-year-old male with history of chronic gastrointestinal dysmotility and chronic back pain treated with opioids presented in septic shock. A CT scan of the abdomen and pelvis, obtained due to worsening abdominal distension, revealed marked thickening of the descending colon wall and hyperdense metallic structures within the colon lumen. Abdominal X-ray confirmed 17 capsule-like objects scattered across the lower quadrants, later identified as retained vibrating capsules.
EGD and colonoscopy revealed two vibrating capsules lodged in the sigmoid colon -- one of which was removed using a Roth net. Several other capsules were embedded in the colonic mucosa but could not be removed endoscopically. The mucosa appeared significantly erythematous and ulcerated, raising concern for ischemic colitis.
Exploratory laparotomy revealed necrotic small bowel with perforation and partially necrotic large bowel with perforation. A small bowel resection and partial colectomy from the right transverse colon to the proximal sigmoid was prefomed. Intraoperatively, several capsules were found to have perforated through the colonic mucosa. The patient was eventually downgraded from the ICU and discharged to a rehab facility after an extended hospital stay.
Discussion:
Vibrating capsules offer a novel approach to managing chronic constipation, but their risks must be weighed carefully, especially in patients with gastrointestinal dysmotility. In this case, 17 capsules were retained in a patient who developed ischemic colitis and bowel perforation. Although causality cannot be confirmed, impaired capsule clearance likely contributed. Vibrating capsules are intended for use up to five times per week and typically pass within three days; significant retention, as seen here, suggests caution is warranted. Further studies are needed to assess safety in high-risk populations, including those with altered GI anatomy or risk of obstruction.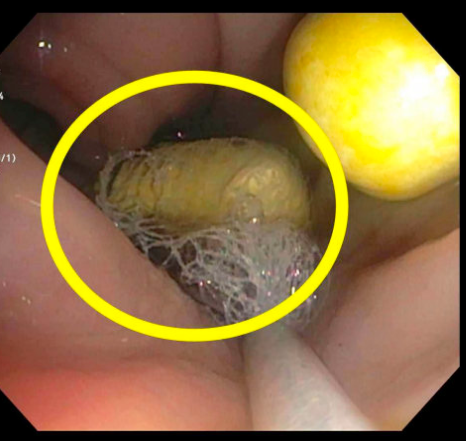
Figure: Endoscopic view of the sigmoid colon demonstrating two retained vibrating capsules
Disclosures:
Charmi Patel indicated no relevant financial relationships.
Rohan Karkra indicated no relevant financial relationships.
Charmi Patel, MD, Rohan Karkra, MBBS. P4626 - Vibrating Capsules: Revolutionizing Constipation Management or Just a Shaky Gimmick?, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
