Tuesday Poster Session
Category: Biliary/Pancreas
P4405 - Pembrolizumab-Induced Sclerosing Cholangitis With Limited Steroid Response
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Rachel McNulty, DO (she/her/hers)
Cleveland Clinic Akron General
Akron, OH
Presenting Author(s)
Rachel McNulty, DO, Vasu Gupta, MD, Abdulfatah Issak, MD
Cleveland Clinic Akron General, Akron, OH
Introduction: Immune checkpoint inhibitor-related sclerosing cholangitis (irSC) is a rare and poorly defined immune-related adverse event (irAE). Reported incidence varies from 0.8% to 4.5%. Clinical presentation ranges from asymptomatic lab abnormalities to abdominal pain and fever indicative of cholangitis. CD8 + T cell mediated inflammation is well-defined, however steroid therapy has inconsistent efficacy. Multiple societies have recommendations for immune checkpoint inhibitor hepatotoxicity; there are none for irSC, despite its prolonged course and associated mortality. Here, we present a case of irSC with limited steroid response.
Case Description/
Methods: A 76-year-old woman with metastatic renal cell carcinoma to the liver, lung and adrenals developed acute liver injury after six pembrolizumab cycles. Labs showed ALP 1,011, AST 444, ALT 794, and bilirubin 1.4. MRCP showed irregular intrahepatic biliary duct dilation with beading, suggestive of irSC. There was no focal biliary ductal obstruction amenable to endoscopic intervention. Prednisone 60 mg daily was started; liver function improved over 2 months. In week 3 of an attempted taper, she reported worsening fatigue and confusion with ALP 702, AST 103, ALT 251, total/direct bilirubin 4.4/2.8. Repeat imaging with CT scan revealed stable intrahepatic biliary dilation. Pembrolizumab was discontinued indefinitely. ALP remains around 500-600 while on prednisone.
Discussion: IrSC is most often linked to PD-1/PD-L1 inhibitor use in older males. Risk factors include autoimmune disease, high-dose or combination therapy, and an underlying diagnosis of melanoma. Onset is insidious, with development of abdominal pain or jaundice over a median of 5.5 cycles. Imaging differentiates irSC from autoimmune hepatitis: CT may show biliary dilation or wall hypertrophy, and MRCP reveals diffuse strictures without large duct obstruction. ERCP often shows pruned-tree bile duct patterns. Hepatotoxicity severity is graded via common terminology criteria for adverse events (CTCAE) to guide initial management. Treatment of irSC starts with glucocorticoids, though response rates are low (11%). UDCA may offer cytoprotective effects but data is limited. Immunomodulators like mycophenolate and tacrolimus show little benefit in few reported cases, and infliximab failed in one instance. Our patient remains on steroids with ongoing evaluation of treatment options. This case highlights the need for diagnostic criteria and management recommendations for irSC.
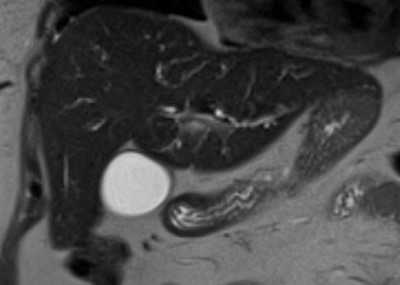
Figure: Image 1. MRCP demonstrating diffuse intrahepatic biliary duct dilation
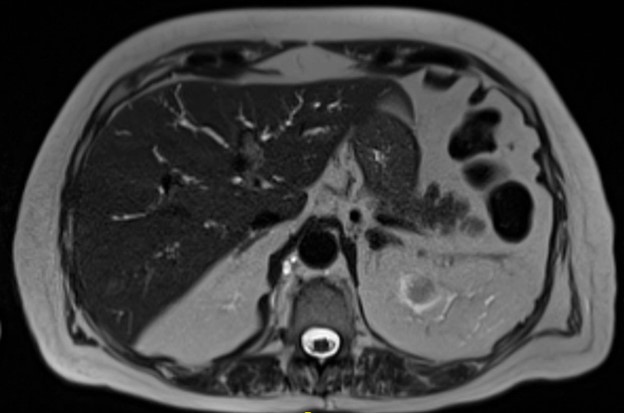
Figure: Image 2. MRCP showing irregular, beaded intrahepatic biliary duct dilation
Disclosures:
Rachel McNulty indicated no relevant financial relationships.
Vasu Gupta indicated no relevant financial relationships.
Abdulfatah Issak indicated no relevant financial relationships.
Rachel McNulty, DO, Vasu Gupta, MD, Abdulfatah Issak, MD. P4405 - Pembrolizumab-Induced Sclerosing Cholangitis With Limited Steroid Response, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Cleveland Clinic Akron General, Akron, OH
Introduction: Immune checkpoint inhibitor-related sclerosing cholangitis (irSC) is a rare and poorly defined immune-related adverse event (irAE). Reported incidence varies from 0.8% to 4.5%. Clinical presentation ranges from asymptomatic lab abnormalities to abdominal pain and fever indicative of cholangitis. CD8 + T cell mediated inflammation is well-defined, however steroid therapy has inconsistent efficacy. Multiple societies have recommendations for immune checkpoint inhibitor hepatotoxicity; there are none for irSC, despite its prolonged course and associated mortality. Here, we present a case of irSC with limited steroid response.
Case Description/
Methods: A 76-year-old woman with metastatic renal cell carcinoma to the liver, lung and adrenals developed acute liver injury after six pembrolizumab cycles. Labs showed ALP 1,011, AST 444, ALT 794, and bilirubin 1.4. MRCP showed irregular intrahepatic biliary duct dilation with beading, suggestive of irSC. There was no focal biliary ductal obstruction amenable to endoscopic intervention. Prednisone 60 mg daily was started; liver function improved over 2 months. In week 3 of an attempted taper, she reported worsening fatigue and confusion with ALP 702, AST 103, ALT 251, total/direct bilirubin 4.4/2.8. Repeat imaging with CT scan revealed stable intrahepatic biliary dilation. Pembrolizumab was discontinued indefinitely. ALP remains around 500-600 while on prednisone.
Discussion: IrSC is most often linked to PD-1/PD-L1 inhibitor use in older males. Risk factors include autoimmune disease, high-dose or combination therapy, and an underlying diagnosis of melanoma. Onset is insidious, with development of abdominal pain or jaundice over a median of 5.5 cycles. Imaging differentiates irSC from autoimmune hepatitis: CT may show biliary dilation or wall hypertrophy, and MRCP reveals diffuse strictures without large duct obstruction. ERCP often shows pruned-tree bile duct patterns. Hepatotoxicity severity is graded via common terminology criteria for adverse events (CTCAE) to guide initial management. Treatment of irSC starts with glucocorticoids, though response rates are low (11%). UDCA may offer cytoprotective effects but data is limited. Immunomodulators like mycophenolate and tacrolimus show little benefit in few reported cases, and infliximab failed in one instance. Our patient remains on steroids with ongoing evaluation of treatment options. This case highlights the need for diagnostic criteria and management recommendations for irSC.

Figure: Image 1. MRCP demonstrating diffuse intrahepatic biliary duct dilation

Figure: Image 2. MRCP showing irregular, beaded intrahepatic biliary duct dilation
Disclosures:
Rachel McNulty indicated no relevant financial relationships.
Vasu Gupta indicated no relevant financial relationships.
Abdulfatah Issak indicated no relevant financial relationships.
Rachel McNulty, DO, Vasu Gupta, MD, Abdulfatah Issak, MD. P4405 - Pembrolizumab-Induced Sclerosing Cholangitis With Limited Steroid Response, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
