Tuesday Poster Session
Category: Biliary/Pancreas
P4310 - Efficacy and Safety of FOLFIRINOX as First-Line Treatment for Advanced Biliary Tract Cancers: A Single-Arm Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Mohammed Y. Youssef, MD
Hunt Regional Medical Center
Greenville, TX
Presenting Author(s)
Mohammed Y. Youssef, MD1, Mohamad Elgozair, MD2, Marina Takawy, MD3, Abdallfatah Abdallfatah, 4, Ahmed N.. Mohamed, MD5, Mohammad Adam, MD, MSc6, Hazem Abosheaishaa, MD7
1Hunt Regional Medical Center, Greenville, TX; 2Danbury / Yale New Haven Hospital, Danbury, CT; 3Rochester Regional Health, Unity Hospital, Rochester, NY; 4Faculty of Medicine, October 6 University, Giza, Al Jizah, Egypt; 5Cleveland Clinic Foundation, South Euclid, OH; 6University of Missouri - Kansas City School of Medicine, Kansas City, MO; 7Mount Sinai West, Icahn School of Medicine at Mount Sinai, Queens, NY
Introduction: Biliary tract cancer (BTC) is a rare, aggressive malignancy with poor prognosis and limited treatment options. Gemcitabine and cisplatin (GemCis) have remained the standard first-line regimen for over a decade. However, response rates are modest, prompting interest in more active regimens. FOLFIRINOX, a triplet chemotherapy regimen originally developed for pancreatic cancer, has shown potential in BTC, but its role is not well defined. This study aimed to evaluate the efficacy and safety of first-line FOLFIRINOX in advanced BTC through a single-arm meta-analysis.
Methods: A systematic review and meta-analysis were conducted, including studies that evaluated FOLFIRINOX as first-line therapy in advanced BTC. A total of 184 patients were analyzed across four studies. The sample included 80 females and 104 males with mean ages ranging from 49.9 to 65.6 years. Primary outcomes were complete response (CR), overall survival (OS), and progression-free survival (PFS). Secondary outcomes included treatment-related adverse events such as anemia, fatigue, and thrombocytopenia. Pooled estimates were generated using a random-effects model, and forest plots were used to assess consistency across studies.
Results: The pooled complete response rate was 2.9% (95% CI: 1.2–6.7%), with all studies showing statistically significant contributions to the overall estimate (Z-values from –2.29 to –5.81, p < 0.05). Mean PFS was 6.66 months (95% CI: 6.59–6.74), with a narrow confidence interval and minimal variance across studies, suggesting consistent disease control. Mean OS was 13.1 months (95% CI: 13.25–21.97). Hematologic toxicity was common: anemia occurred in 71.4% (95% CI: 63–78.6%), fatigue in 67.7% (95% CI: 58.3–75.9%), and thrombocytopenia in 61.8% (95% CI: 53.5–69.5%). These adverse events were largely grade ≥2, highlighting a need for careful supportive care during treatment.
Discussion: FOLFIRINOX demonstrates modest but consistent survival benefits in patients with advanced BTC, with a low rate of complete response but meaningful disease stabilization. However, the high incidence of hematologic toxicity emphasizes the importance of patient selection and monitoring. These findings suggest that FOLFIRINOX may be a viable first-line option for fit patients with advanced BTC, but further prospective studies are needed to confirm these results and identify which patients are most likely to benefit
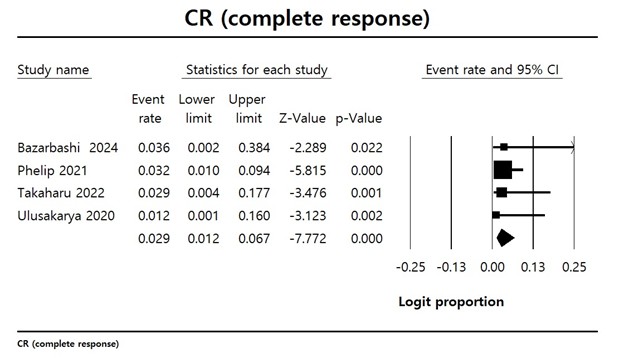
Figure: Figure 1. Forest plot with the result of single-arm meta-analysis for complete response rate of patients with Biliary tract cancer

Figure: Figure 2. Forest plot with the result of single-arm meta-analysis of the mean Progression free survival for patients with Biliary tract cancer
Disclosures:
Mohammed Y. Youssef indicated no relevant financial relationships.
Mohamad Elgozair indicated no relevant financial relationships.
Marina Takawy indicated no relevant financial relationships.
Abdallfatah Abdallfatah indicated no relevant financial relationships.
Ahmed Mohamed indicated no relevant financial relationships.
Mohammad Adam indicated no relevant financial relationships.
Hazem Abosheaishaa indicated no relevant financial relationships.
Mohammed Y. Youssef, MD1, Mohamad Elgozair, MD2, Marina Takawy, MD3, Abdallfatah Abdallfatah, 4, Ahmed N.. Mohamed, MD5, Mohammad Adam, MD, MSc6, Hazem Abosheaishaa, MD7. P4310 - Efficacy and Safety of FOLFIRINOX as First-Line Treatment for Advanced Biliary Tract Cancers: A Single-Arm Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Hunt Regional Medical Center, Greenville, TX; 2Danbury / Yale New Haven Hospital, Danbury, CT; 3Rochester Regional Health, Unity Hospital, Rochester, NY; 4Faculty of Medicine, October 6 University, Giza, Al Jizah, Egypt; 5Cleveland Clinic Foundation, South Euclid, OH; 6University of Missouri - Kansas City School of Medicine, Kansas City, MO; 7Mount Sinai West, Icahn School of Medicine at Mount Sinai, Queens, NY
Introduction: Biliary tract cancer (BTC) is a rare, aggressive malignancy with poor prognosis and limited treatment options. Gemcitabine and cisplatin (GemCis) have remained the standard first-line regimen for over a decade. However, response rates are modest, prompting interest in more active regimens. FOLFIRINOX, a triplet chemotherapy regimen originally developed for pancreatic cancer, has shown potential in BTC, but its role is not well defined. This study aimed to evaluate the efficacy and safety of first-line FOLFIRINOX in advanced BTC through a single-arm meta-analysis.
Methods: A systematic review and meta-analysis were conducted, including studies that evaluated FOLFIRINOX as first-line therapy in advanced BTC. A total of 184 patients were analyzed across four studies. The sample included 80 females and 104 males with mean ages ranging from 49.9 to 65.6 years. Primary outcomes were complete response (CR), overall survival (OS), and progression-free survival (PFS). Secondary outcomes included treatment-related adverse events such as anemia, fatigue, and thrombocytopenia. Pooled estimates were generated using a random-effects model, and forest plots were used to assess consistency across studies.
Results: The pooled complete response rate was 2.9% (95% CI: 1.2–6.7%), with all studies showing statistically significant contributions to the overall estimate (Z-values from –2.29 to –5.81, p < 0.05). Mean PFS was 6.66 months (95% CI: 6.59–6.74), with a narrow confidence interval and minimal variance across studies, suggesting consistent disease control. Mean OS was 13.1 months (95% CI: 13.25–21.97). Hematologic toxicity was common: anemia occurred in 71.4% (95% CI: 63–78.6%), fatigue in 67.7% (95% CI: 58.3–75.9%), and thrombocytopenia in 61.8% (95% CI: 53.5–69.5%). These adverse events were largely grade ≥2, highlighting a need for careful supportive care during treatment.
Discussion: FOLFIRINOX demonstrates modest but consistent survival benefits in patients with advanced BTC, with a low rate of complete response but meaningful disease stabilization. However, the high incidence of hematologic toxicity emphasizes the importance of patient selection and monitoring. These findings suggest that FOLFIRINOX may be a viable first-line option for fit patients with advanced BTC, but further prospective studies are needed to confirm these results and identify which patients are most likely to benefit

Figure: Figure 1. Forest plot with the result of single-arm meta-analysis for complete response rate of patients with Biliary tract cancer

Figure: Figure 2. Forest plot with the result of single-arm meta-analysis of the mean Progression free survival for patients with Biliary tract cancer
Disclosures:
Mohammed Y. Youssef indicated no relevant financial relationships.
Mohamad Elgozair indicated no relevant financial relationships.
Marina Takawy indicated no relevant financial relationships.
Abdallfatah Abdallfatah indicated no relevant financial relationships.
Ahmed Mohamed indicated no relevant financial relationships.
Mohammad Adam indicated no relevant financial relationships.
Hazem Abosheaishaa indicated no relevant financial relationships.
Mohammed Y. Youssef, MD1, Mohamad Elgozair, MD2, Marina Takawy, MD3, Abdallfatah Abdallfatah, 4, Ahmed N.. Mohamed, MD5, Mohammad Adam, MD, MSc6, Hazem Abosheaishaa, MD7. P4310 - Efficacy and Safety of FOLFIRINOX as First-Line Treatment for Advanced Biliary Tract Cancers: A Single-Arm Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
