Tuesday Poster Session
Category: Biliary/Pancreas
P4304 - The Use of Pancreatic Enzyme Replacement Therapy During Chemotherapy for Pancreatic Cancer: A Retrospective Cross-Sectional Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Amar Farkouh, MD (he/him/his)
University of Montreal
Montreal, QC, Canada
Presenting Author(s)
Emilie Kate. Landry, MD, Amar Farkouh, MD, Roy Hajjar, MD, PhD, MSc(Epi), Gabriel Chan, MD
University of Montreal, Montreal, PQ, Canada
Introduction: Pancreatic cancer (PC) is a devastating diagnosis, often presenting at an advanced stage. Pancreatic exocrine insufficiency (PEI) is commonly associated, due to tissue destruction, Wirsung duct occlusion and surgical resection. PEI can be easily overlooked amidst the shock and overlapping symptoms with advanced cancer or the side effects of chemotherapy. Pancreatic enzyme replacement therapy (PERT) has proven to be beneficial during chemotherapy. The study objectives are to determine the use of PERT during chemotherapy and possible associations with outcomes.
Methods: A retrospective cross-sectional study was conducted at a Canadian university-affiliated regional oncology centre. All medical records of patients initiating a chemotherapy regimen for PC between January 1, 2019 and September 30, 2023, were reviewed.
Results: The study population included 100 PC patients with a median age of 68 years. Demographic data and clinical course are presented in Table 1. Chemotherapy was administered in neoadjuvant, adjuvant, and palliative settings for 17, 19, and 64 patients, respectively. First-line regimens included FOLFIRINOX (39%), gemcitabine alone (40%), nab-paclitaxel-gemcitabine (20%), or other (1%). Twenty-five patients had a complete oncological resection, of which five had neoadjuvant chemotherapy. Thirty-six patients received a second-line regimen, and 12 received three or more regimens. PERT was prescribed in only 17% of patients with palliative chemotherapy, despite the majority (83%) having had signs of PEI (weight loss, diarrhea or obstructed Wirsung duct with dilatation). There were no significant differences associated with weight changes, number of cycles of chemotherapy, interruptions of regimen protocols, nor survival when comparing PERT status in the palliative group. The highest prevalence of PERT prescription (42%) was in the adjuvant chemotherapy group. No patients in the neoadjuvant group received PERT during chemotherapy.
Discussion: In this retrospective review of clinical practice patterns, PEI seems to be overlooked, and PERT is underutilized for PC patients during palliative and neoadjuvant chemotherapy. Barriers include uncertainties about the diagnostic approach of PEI, lack of awareness of PERT as nutritional adjunctive therapy and lack of patient education and a multidisciplinary approach. Future prospective studies should include PERT as an adjunct to neoadjuvant chemotherapy and formally measured symptoms of PEI.
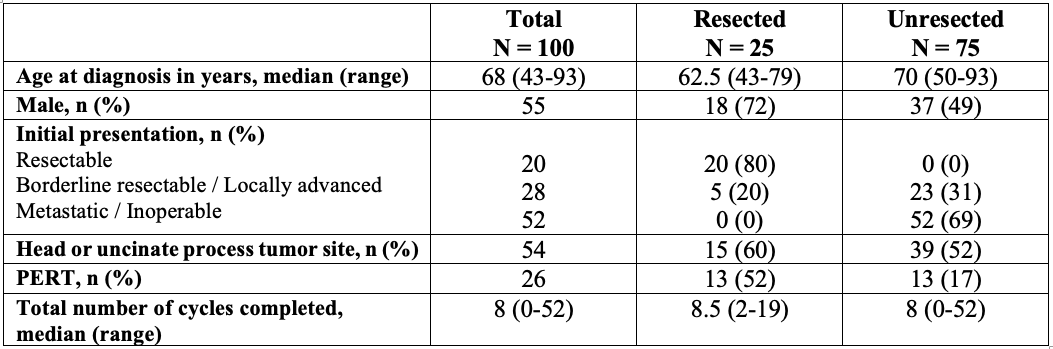
Figure: Table 1. Demographic information and clinical course
Disclosures:
Emilie Landry indicated no relevant financial relationships.
Amar Farkouh indicated no relevant financial relationships.
Roy Hajjar indicated no relevant financial relationships.
Gabriel Chan: Paladin Pharma – Advisory Committee/Board Member. Viatris – Speakers Bureau.
Emilie Kate. Landry, MD, Amar Farkouh, MD, Roy Hajjar, MD, PhD, MSc(Epi), Gabriel Chan, MD. P4304 - The Use of Pancreatic Enzyme Replacement Therapy During Chemotherapy for Pancreatic Cancer: A Retrospective Cross-Sectional Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
University of Montreal, Montreal, PQ, Canada
Introduction: Pancreatic cancer (PC) is a devastating diagnosis, often presenting at an advanced stage. Pancreatic exocrine insufficiency (PEI) is commonly associated, due to tissue destruction, Wirsung duct occlusion and surgical resection. PEI can be easily overlooked amidst the shock and overlapping symptoms with advanced cancer or the side effects of chemotherapy. Pancreatic enzyme replacement therapy (PERT) has proven to be beneficial during chemotherapy. The study objectives are to determine the use of PERT during chemotherapy and possible associations with outcomes.
Methods: A retrospective cross-sectional study was conducted at a Canadian university-affiliated regional oncology centre. All medical records of patients initiating a chemotherapy regimen for PC between January 1, 2019 and September 30, 2023, were reviewed.
Results: The study population included 100 PC patients with a median age of 68 years. Demographic data and clinical course are presented in Table 1. Chemotherapy was administered in neoadjuvant, adjuvant, and palliative settings for 17, 19, and 64 patients, respectively. First-line regimens included FOLFIRINOX (39%), gemcitabine alone (40%), nab-paclitaxel-gemcitabine (20%), or other (1%). Twenty-five patients had a complete oncological resection, of which five had neoadjuvant chemotherapy. Thirty-six patients received a second-line regimen, and 12 received three or more regimens. PERT was prescribed in only 17% of patients with palliative chemotherapy, despite the majority (83%) having had signs of PEI (weight loss, diarrhea or obstructed Wirsung duct with dilatation). There were no significant differences associated with weight changes, number of cycles of chemotherapy, interruptions of regimen protocols, nor survival when comparing PERT status in the palliative group. The highest prevalence of PERT prescription (42%) was in the adjuvant chemotherapy group. No patients in the neoadjuvant group received PERT during chemotherapy.
Discussion: In this retrospective review of clinical practice patterns, PEI seems to be overlooked, and PERT is underutilized for PC patients during palliative and neoadjuvant chemotherapy. Barriers include uncertainties about the diagnostic approach of PEI, lack of awareness of PERT as nutritional adjunctive therapy and lack of patient education and a multidisciplinary approach. Future prospective studies should include PERT as an adjunct to neoadjuvant chemotherapy and formally measured symptoms of PEI.

Figure: Table 1. Demographic information and clinical course
Disclosures:
Emilie Landry indicated no relevant financial relationships.
Amar Farkouh indicated no relevant financial relationships.
Roy Hajjar indicated no relevant financial relationships.
Gabriel Chan: Paladin Pharma – Advisory Committee/Board Member. Viatris – Speakers Bureau.
Emilie Kate. Landry, MD, Amar Farkouh, MD, Roy Hajjar, MD, PhD, MSc(Epi), Gabriel Chan, MD. P4304 - The Use of Pancreatic Enzyme Replacement Therapy During Chemotherapy for Pancreatic Cancer: A Retrospective Cross-Sectional Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

