Sunday Poster Session
Category: Liver
P1816 - Hepatic Peliosis: Not Just a Theoretical Risk of Testosterone Use in Gender-Affirming Hormone Therapy
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Taylor Seacor, MD
University of Massachusetts Chan Medical School
Worcester, MA
Presenting Author(s)
Taylor Seacor, MD1, Sankirth Madabhushi, MD1, Courtney Sanders, DO1, Garrick Gu, MD1, Anita Krishnarao, MD2
1University of Massachusetts Chan Medical School, Worcester, MA; 2University of Massachusetts Memorial Health, Worcester, MA
Introduction: Peliosis hepatis is a rare vascular condition of the liver in which the sinusoids of the liver proliferate resulting in capillary bed engorgement and blood-filled cystic cavities. Hepatic peliosis has been associated with medications like immunosuppressants and methyltestosterone. Here we present a unique case of peliosis hepatis following testosterone cypionate administration as part of masculinizing hormone therapy.
Case Description/
Methods: Patient is a 22 year old transgender female to male with a past medical history of euthyroid hashimoto’s thyroiditis with normal liver function that was initiated on testosterone cypionate intramuscular injections in May 2019. Patient was noted to have uptrending LFTs four months after the first injection with ALT/AST 49. The patient underwent liver biopsy in September 2020, revealing sinusoidal dilation, focal atrophy of the liver cell plates, and focal cystic spaces with blood cells. Testosterone cypionate was discontinued in September 2020. After extensive risk vs benefit conversion, the patient elected to start transdermal testosterone gel (Androgel) in February 2021 with return to baseline liver chemistries in 2022 (Figure 1).
Discussion: While peliosis hepatis has been previously linked to certain anabolic steroids, particularly oral alkylated androgens such as methyltestosterone, its occurrence with other testosterone formulations, including testosterone cypionate or transdermal testosterone is not well documented in literature. Hence, hepatic peliosis has been described as a theoretical risk for exogenous testosterone. Peliosis hepatis typically resolves following the discontinuation of the implicated medications, which affirms the importance of early identification. This patient’s liver function improved when switched from intramuscular testosterone cypionate to transdermal testosterone. The improvement in liver function can likely be attributed to bypassing the liver’s first-pass metabolism and should be considered in patients with high risk of hepatotoxicity undergoing masculinizing hormone therapy. Further studies are required to focus on hepatotoxicity in gender-affirming therapy and the association between hepatic peliosis and differing formulations of testosterone therapy.
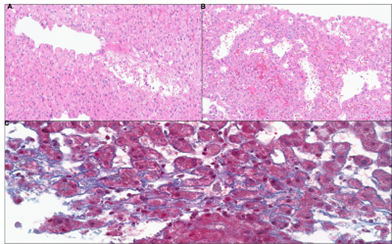
Figure: Figure 1. Timeline of liver function tests
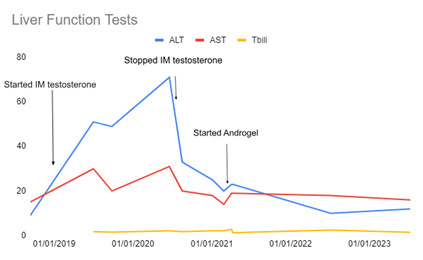
Figure: Figure 2. Histology of a liver biopsy with peliosis-like changes consisting of (A-B) marked blood-filled endothelial-lined sinusoidal dilation (H&E, X150) and (C) mild perisinusoidal fibrosis (Trichrome, X300)
Disclosures:
Taylor Seacor indicated no relevant financial relationships.
Sankirth Madabhushi indicated no relevant financial relationships.
Courtney Sanders indicated no relevant financial relationships.
Garrick Gu indicated no relevant financial relationships.
Anita Krishnarao: Madrigal Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau.
Taylor Seacor, MD1, Sankirth Madabhushi, MD1, Courtney Sanders, DO1, Garrick Gu, MD1, Anita Krishnarao, MD2. P1816 - Hepatic Peliosis: Not Just a Theoretical Risk of Testosterone Use in Gender-Affirming Hormone Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Massachusetts Chan Medical School, Worcester, MA; 2University of Massachusetts Memorial Health, Worcester, MA
Introduction: Peliosis hepatis is a rare vascular condition of the liver in which the sinusoids of the liver proliferate resulting in capillary bed engorgement and blood-filled cystic cavities. Hepatic peliosis has been associated with medications like immunosuppressants and methyltestosterone. Here we present a unique case of peliosis hepatis following testosterone cypionate administration as part of masculinizing hormone therapy.
Case Description/
Methods: Patient is a 22 year old transgender female to male with a past medical history of euthyroid hashimoto’s thyroiditis with normal liver function that was initiated on testosterone cypionate intramuscular injections in May 2019. Patient was noted to have uptrending LFTs four months after the first injection with ALT/AST 49. The patient underwent liver biopsy in September 2020, revealing sinusoidal dilation, focal atrophy of the liver cell plates, and focal cystic spaces with blood cells. Testosterone cypionate was discontinued in September 2020. After extensive risk vs benefit conversion, the patient elected to start transdermal testosterone gel (Androgel) in February 2021 with return to baseline liver chemistries in 2022 (Figure 1).
Discussion: While peliosis hepatis has been previously linked to certain anabolic steroids, particularly oral alkylated androgens such as methyltestosterone, its occurrence with other testosterone formulations, including testosterone cypionate or transdermal testosterone is not well documented in literature. Hence, hepatic peliosis has been described as a theoretical risk for exogenous testosterone. Peliosis hepatis typically resolves following the discontinuation of the implicated medications, which affirms the importance of early identification. This patient’s liver function improved when switched from intramuscular testosterone cypionate to transdermal testosterone. The improvement in liver function can likely be attributed to bypassing the liver’s first-pass metabolism and should be considered in patients with high risk of hepatotoxicity undergoing masculinizing hormone therapy. Further studies are required to focus on hepatotoxicity in gender-affirming therapy and the association between hepatic peliosis and differing formulations of testosterone therapy.

Figure: Figure 1. Timeline of liver function tests

Figure: Figure 2. Histology of a liver biopsy with peliosis-like changes consisting of (A-B) marked blood-filled endothelial-lined sinusoidal dilation (H&E, X150) and (C) mild perisinusoidal fibrosis (Trichrome, X300)
Disclosures:
Taylor Seacor indicated no relevant financial relationships.
Sankirth Madabhushi indicated no relevant financial relationships.
Courtney Sanders indicated no relevant financial relationships.
Garrick Gu indicated no relevant financial relationships.
Anita Krishnarao: Madrigal Pharmaceuticals – Advisory Committee/Board Member, Speakers Bureau.
Taylor Seacor, MD1, Sankirth Madabhushi, MD1, Courtney Sanders, DO1, Garrick Gu, MD1, Anita Krishnarao, MD2. P1816 - Hepatic Peliosis: Not Just a Theoretical Risk of Testosterone Use in Gender-Affirming Hormone Therapy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

