Sunday Poster Session
Category: Liver
P1808 - Reishi Mushroom Toxicity in Chronic Liver Disease
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Anh Bui, DO (she/her/hers)
Cleveland Clinic Foundation
Cleveland, OH
Presenting Author(s)
Anh Bui, DO1, Garrett Cotter, MD1, Saleh Alghsoon, MBBS2, Kenneth Friedman, MD3, Dian Jung Chiang, MD3
1Cleveland Clinic Foundation, Cleveland, OH; 2Cleveland clinic, Cleveland, OH; 3Cleveland Clinic, Cleveland, OH
Introduction: Ganoderma lingzhi (the Reishi mushroom) has become a popular supplement that boosts the immune system, with claimed antioxidant properties. However, there is limited data on its safety and potential liver toxicity.
Case Description/
Methods: A 51-year-old woman with a history of alcohol use disorder presented for abnormal liver enzymes. She had a recent hospitalization for acute liver injury requiring N-acetylcysteine. She endorsed progressive fatigue and abdominal pain with labs showing abnormal liver enzymes with hepatocellular injury pattern and impaired liver synthetic function. An extensive serological work up for chronic and acute liver disease was negative for viral hepatitis, metabolic and genetic liver diseases. She received NAC and supportive care with initial improvement in aminotransferases. Her total bilirubin continued to increase during this admission. She underwent a liver biopsy showing bridging fibrosis, minimal interface change, variable macrovesicular steatosis, and zone III hepatocellular collapse suggestive of potential drug induced liver injury-autoimmune hepatitis. Two weeks after discharge, she returned with worsening abdominal pain, jaundice, with worsening cholestatic liver injury. Upon detailed review she endorsed long-term use of Lingzhi powder for a duration of three months. The last consumption was two months prior to the initial presentation. She also reported daily alcohol consumption. Medical therapy of prednisone taper, azathioprine, and ursodiol was initiated. The patient liver function test continued to improve over the course on azathioprine upon outpatient follow-up at two months after discharge.
Discussion: Ganoderma lingzhi, commonly known as reishi mushroom, has seen a surge in popularity as a natural health supplement, with U.S. sales reaching $3.6 million in 2019. The increase in reishi mushroom consumption over the years is paralleled by a growing number of drug-induced liver injury cases (DILI), which now account for over 20% of all reported liver injury. This is the second documented case of Reishi mushroom related DILI in patients with underlying alcoholic liver disease. The incidence and risk factors for Ganoderma genus induced liver injury is limited and complicated by conflicting clinical data. Ganoderma lucidum—a different form—has shown hepatoprotective effects in animal studies, particularly through modulation of the TLR4/NF-kB/MyD88 signaling pathway. This case underscores the importance of herbal supplements in chronic liver disease.
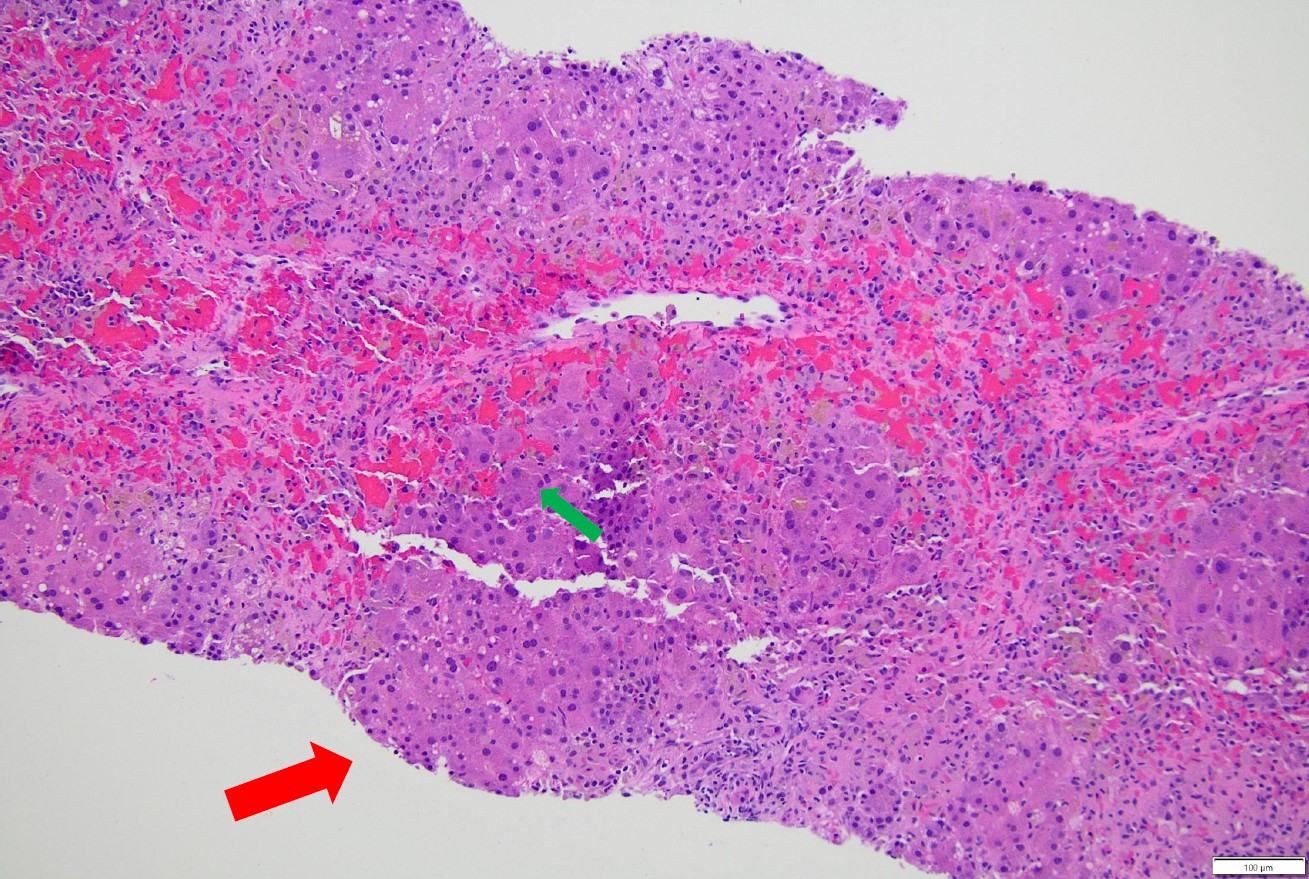
Figure: There is a nodule of viable hepatocytes with a partial portal tract (red arrow). However, surrounding this nodule is an area of hepatocyte necrosis and hemorrhage (green arrow). The absence of hepatocytes has "collapsed" the overall architecture, so that areas of necrosis are connecting to each other, what we refer to as "bridging necrosis". Bridging necrosis can be seen in chronic liver injury. In combination with extension of fibrosis from the portal tracts, referred to as interface hepatitis, which is consistent with DILI-AIH.
Disclosures:
Anh Bui indicated no relevant financial relationships.
Garrett Cotter indicated no relevant financial relationships.
Saleh Alghsoon indicated no relevant financial relationships.
Kenneth Friedman indicated no relevant financial relationships.
Dian Jung Chiang: Ipsen – Advisory Committee/Board Member.
Anh Bui, DO1, Garrett Cotter, MD1, Saleh Alghsoon, MBBS2, Kenneth Friedman, MD3, Dian Jung Chiang, MD3. P1808 - Reishi Mushroom Toxicity in Chronic Liver Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Cleveland Clinic Foundation, Cleveland, OH; 2Cleveland clinic, Cleveland, OH; 3Cleveland Clinic, Cleveland, OH
Introduction: Ganoderma lingzhi (the Reishi mushroom) has become a popular supplement that boosts the immune system, with claimed antioxidant properties. However, there is limited data on its safety and potential liver toxicity.
Case Description/
Methods: A 51-year-old woman with a history of alcohol use disorder presented for abnormal liver enzymes. She had a recent hospitalization for acute liver injury requiring N-acetylcysteine. She endorsed progressive fatigue and abdominal pain with labs showing abnormal liver enzymes with hepatocellular injury pattern and impaired liver synthetic function. An extensive serological work up for chronic and acute liver disease was negative for viral hepatitis, metabolic and genetic liver diseases. She received NAC and supportive care with initial improvement in aminotransferases. Her total bilirubin continued to increase during this admission. She underwent a liver biopsy showing bridging fibrosis, minimal interface change, variable macrovesicular steatosis, and zone III hepatocellular collapse suggestive of potential drug induced liver injury-autoimmune hepatitis. Two weeks after discharge, she returned with worsening abdominal pain, jaundice, with worsening cholestatic liver injury. Upon detailed review she endorsed long-term use of Lingzhi powder for a duration of three months. The last consumption was two months prior to the initial presentation. She also reported daily alcohol consumption. Medical therapy of prednisone taper, azathioprine, and ursodiol was initiated. The patient liver function test continued to improve over the course on azathioprine upon outpatient follow-up at two months after discharge.
Discussion: Ganoderma lingzhi, commonly known as reishi mushroom, has seen a surge in popularity as a natural health supplement, with U.S. sales reaching $3.6 million in 2019. The increase in reishi mushroom consumption over the years is paralleled by a growing number of drug-induced liver injury cases (DILI), which now account for over 20% of all reported liver injury. This is the second documented case of Reishi mushroom related DILI in patients with underlying alcoholic liver disease. The incidence and risk factors for Ganoderma genus induced liver injury is limited and complicated by conflicting clinical data. Ganoderma lucidum—a different form—has shown hepatoprotective effects in animal studies, particularly through modulation of the TLR4/NF-kB/MyD88 signaling pathway. This case underscores the importance of herbal supplements in chronic liver disease.

Figure: There is a nodule of viable hepatocytes with a partial portal tract (red arrow). However, surrounding this nodule is an area of hepatocyte necrosis and hemorrhage (green arrow). The absence of hepatocytes has "collapsed" the overall architecture, so that areas of necrosis are connecting to each other, what we refer to as "bridging necrosis". Bridging necrosis can be seen in chronic liver injury. In combination with extension of fibrosis from the portal tracts, referred to as interface hepatitis, which is consistent with DILI-AIH.
Disclosures:
Anh Bui indicated no relevant financial relationships.
Garrett Cotter indicated no relevant financial relationships.
Saleh Alghsoon indicated no relevant financial relationships.
Kenneth Friedman indicated no relevant financial relationships.
Dian Jung Chiang: Ipsen – Advisory Committee/Board Member.
Anh Bui, DO1, Garrett Cotter, MD1, Saleh Alghsoon, MBBS2, Kenneth Friedman, MD3, Dian Jung Chiang, MD3. P1808 - Reishi Mushroom Toxicity in Chronic Liver Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
