Sunday Poster Session
Category: Liver
P1806 - Rapid-Onset Liver Failure After First Risankizumab Infusion: Successful Treatment With N-Acetylcysteine
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Saud Khan, MD (he/him/his)
University of Central Florida, HCA Healthcare GME
Orlando, FL
Presenting Author(s)
Award: ACG Presidential Poster Award
Saud Khan, MD, Eric Chen, MD, Ali Ashraf, MD
University of Central Florida, HCA Healthcare GME, Orlando, FL
Introduction: Drug-induced liver injury (DILI) accounts for a considerable proportion of acute liver failure in the United States. While many biologics are associated with immune mediated or idiosyncratic reactions, Risankizumab has not been implicated in severe liver or cardiac injury. Immune modulation in the setting of comorbid conditions may exacerbate susceptibility to hepatic dysfunction.
Case Description/
Methods: A 47-year-old man with a history of Crohn’s disease, metabolic syndrome, stable heart failure with reduced ejection fraction (HFrEF), atrial fibrillation and prior ischemic strokes presented with acute nausea, vomiting and abdominal pain following his first infusion of Risankizumab administered the day prior. He denied any new medications or any family history of liver disease. He was afebrile and hemodynamically stable, with an acute kidney injury and elevated transaminases (Fig 1). Viral hepatitis panel and autoimmune hepatitis serologies were negative. He was initially treated for presumed Crohn’s flare. On day 3, he became unresponsive and hypotensive, prompting intubation and vasopressor support. A liver ultrasound showed hepatomegaly, but patent hepatic vasculature and a common bile duct measuring 6 mm, with no intra or extrahepatic ductal abnormality. Dobutamine and N-acetylcysteine was added, and steroids continued. Over the next 72 hours, his transaminases and INR trended down, encephalopathy improved, and pressors weaned off. His neurological status and hepatic profile returned to baseline and he was discharged on day 13.
Discussion: There are no prior reports of Risankizumab-induced acute liver failure at the time of this writing, although elevated transaminases and cirrhosis have been reported. The pathophysiology remains unclear. Potential mechanisms for acute liver injury include immune-mediated injury, cytokine dysregulation superimposed on chronic hepatic hypoperfusion (underlying HFrEF), or IL-23 mediated cardiac toxicity. However, IL-23 inhibitors are not implicated in cardiac damage. While biologics like Infliximab and Adalimumab have more documented hepatic adverse events, Risankizumab's tertiary data is evolving. Our patient’s negative viral and autoimmune workup, along with the absence of other hepatotoxic exposures lend support to DILI. N-acetylcysteine has been shown to improve outcomes in non-acetaminophen acute liver failure and was used here effectively. With inpatient data, patient scored 5 on the Naranjo scale, indicating possible relation to prior Il-23 infusion.
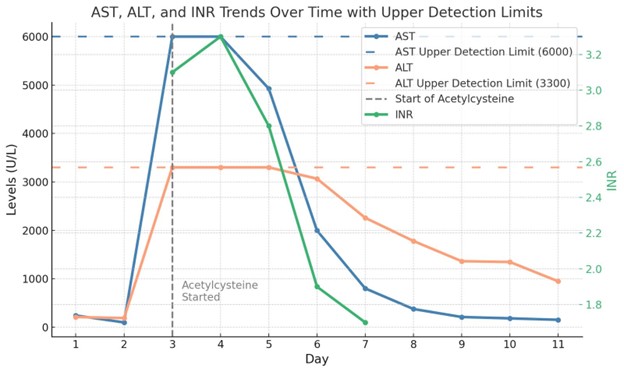
Figure: Fig 1: Trend of hepatic function testing.
Disclosures:
Saud Khan indicated no relevant financial relationships.
Eric Chen indicated no relevant financial relationships.
Ali Ashraf indicated no relevant financial relationships.
Saud Khan, MD, Eric Chen, MD, Ali Ashraf, MD. P1806 - Rapid-Onset Liver Failure After First Risankizumab Infusion: Successful Treatment With N-Acetylcysteine, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Saud Khan, MD, Eric Chen, MD, Ali Ashraf, MD
University of Central Florida, HCA Healthcare GME, Orlando, FL
Introduction: Drug-induced liver injury (DILI) accounts for a considerable proportion of acute liver failure in the United States. While many biologics are associated with immune mediated or idiosyncratic reactions, Risankizumab has not been implicated in severe liver or cardiac injury. Immune modulation in the setting of comorbid conditions may exacerbate susceptibility to hepatic dysfunction.
Case Description/
Methods: A 47-year-old man with a history of Crohn’s disease, metabolic syndrome, stable heart failure with reduced ejection fraction (HFrEF), atrial fibrillation and prior ischemic strokes presented with acute nausea, vomiting and abdominal pain following his first infusion of Risankizumab administered the day prior. He denied any new medications or any family history of liver disease. He was afebrile and hemodynamically stable, with an acute kidney injury and elevated transaminases (Fig 1). Viral hepatitis panel and autoimmune hepatitis serologies were negative. He was initially treated for presumed Crohn’s flare. On day 3, he became unresponsive and hypotensive, prompting intubation and vasopressor support. A liver ultrasound showed hepatomegaly, but patent hepatic vasculature and a common bile duct measuring 6 mm, with no intra or extrahepatic ductal abnormality. Dobutamine and N-acetylcysteine was added, and steroids continued. Over the next 72 hours, his transaminases and INR trended down, encephalopathy improved, and pressors weaned off. His neurological status and hepatic profile returned to baseline and he was discharged on day 13.
Discussion: There are no prior reports of Risankizumab-induced acute liver failure at the time of this writing, although elevated transaminases and cirrhosis have been reported. The pathophysiology remains unclear. Potential mechanisms for acute liver injury include immune-mediated injury, cytokine dysregulation superimposed on chronic hepatic hypoperfusion (underlying HFrEF), or IL-23 mediated cardiac toxicity. However, IL-23 inhibitors are not implicated in cardiac damage. While biologics like Infliximab and Adalimumab have more documented hepatic adverse events, Risankizumab's tertiary data is evolving. Our patient’s negative viral and autoimmune workup, along with the absence of other hepatotoxic exposures lend support to DILI. N-acetylcysteine has been shown to improve outcomes in non-acetaminophen acute liver failure and was used here effectively. With inpatient data, patient scored 5 on the Naranjo scale, indicating possible relation to prior Il-23 infusion.

Figure: Fig 1: Trend of hepatic function testing.
Disclosures:
Saud Khan indicated no relevant financial relationships.
Eric Chen indicated no relevant financial relationships.
Ali Ashraf indicated no relevant financial relationships.
Saud Khan, MD, Eric Chen, MD, Ali Ashraf, MD. P1806 - Rapid-Onset Liver Failure After First Risankizumab Infusion: Successful Treatment With N-Acetylcysteine, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

