Sunday Poster Session
Category: Liver
P1738 - Risk of Severe Hepatotoxicity With Long-Acting Antiretroviral Therapy and Concomitant Acute Hepatitis B Infection: A Rare Clinical Observation
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- PJ
Patricia D. Jones, MD, MSCR
University of Miami Miller School of Medicine
Miami, FL
Presenting Author(s)
Keri-Ann Buchanan-Peart, MD1, Soe H. Arker, MD2, Patricia D. Jones, MD, MSCR3
1University of Miami/Jackson Memorial Hospital, Miami, FL; 2University of Miami/Jackson Health System, Miami, FL; 3University of Miami Miller School of Medicine, Miami, FL
Introduction: Long-acting antiretroviral therapy (ART), Cabenuva, a combination of cabotegravir and rilpivirine, is used to treat HIV-1 infection, with few reports of mild hepatotoxicity in clinical trials. This case highlights the potential for severe hepatotoxicity associated with this long-acting agent, exacerbated by acute hepatitis B virus (HBV) infection.
Case Description/
Methods: A 54-year-old male with HIV (CD4 394/uL) presented with jaundice and acute HBV infection. He was maintained on Cabenuva and monitored regularly. Hepatitis B serologies nine months prior were negative, and liver enzymes were normal. Labs now showed aspartate aminotransferase (AST) 2904 U/L, alanine aminotransferase (ALT) 3995 U/L, bilirubin 11.2 mg/dL, INR 1.1, and acute HBV infection (HBsAg and HBeAg positive, HBV DNA 42.6 million IU/mL). Cabenuva was held due to marked hepatocellular liver injury. Despite modest improvements in liver enzyme levels and HBV viral load (560 IU/mL), bilirubin levels worsened with synthetic dysfunction, anorexia, and weight loss. No hepatic mass or biliary dilation was seen on imaging. Given clinical worsening, Biktarvy, effective against both infections, was started.
Additional testing showed a ferritin level of 6,021 ng/ml with negative serologies for Hepatitis D, E, EBV, and CMV. A liver biopsy revealed acute hepatitis with cholestasis, parenchymal collapse, and stage 4 fibrosis. (Figure 1) CT revealed developing cirrhosis and ascites. Upper endoscopy revealed large, non-bleeding esophageal varices treated with band ligation. Despite HBV suppression, hepatic inflammation persisted, resulting in fibrosis and new-onset portal hypertension. A corticosteroid taper trial showed no benefit. The findings suggest severe hepatotoxicity due to Cabenuva, compounded by acute HBV infection.
Discussion: Cabenuva is well tolerated, with a few cases of mild hepatotoxicity documented. This patient had severe liver injury despite HBV infection control, suggesting the synergistic effect of acute HBV and drug toxicity. The extended half-lives of cabotegravir and rilpivirine (~40 days and 38–43 days, respectively) with measurable levels up to a year post-discontinuation increase the risk of continued liver injury. HBV screening and vaccination are recommended before initiating long-acting ART. Given the increased risk of severe hepatotoxicity with Cabenuva, particularly in the context of acute HBV infection, the need for long-term safety studies for injectable ART is paramount.
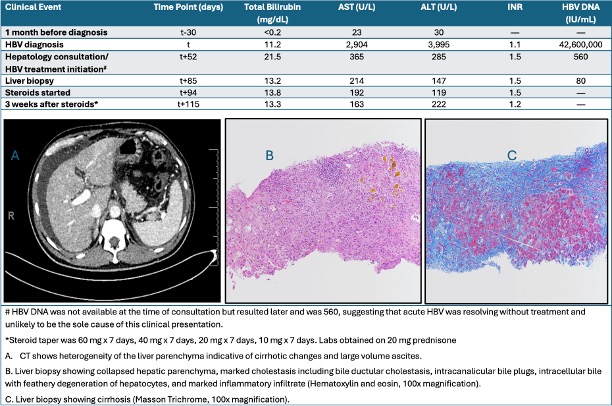
Figure: Figure 1. Lab trends correlating with clinical events; CT abdomen and liver histology showing changes consistent with cirrhosis.
Disclosures:
Keri-Ann Buchanan-Peart indicated no relevant financial relationships.
Soe Arker indicated no relevant financial relationships.
Patricia Jones indicated no relevant financial relationships.
Keri-Ann Buchanan-Peart, MD1, Soe H. Arker, MD2, Patricia D. Jones, MD, MSCR3. P1738 - Risk of Severe Hepatotoxicity With Long-Acting Antiretroviral Therapy and Concomitant Acute Hepatitis B Infection: A Rare Clinical Observation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Miami/Jackson Memorial Hospital, Miami, FL; 2University of Miami/Jackson Health System, Miami, FL; 3University of Miami Miller School of Medicine, Miami, FL
Introduction: Long-acting antiretroviral therapy (ART), Cabenuva, a combination of cabotegravir and rilpivirine, is used to treat HIV-1 infection, with few reports of mild hepatotoxicity in clinical trials. This case highlights the potential for severe hepatotoxicity associated with this long-acting agent, exacerbated by acute hepatitis B virus (HBV) infection.
Case Description/
Methods: A 54-year-old male with HIV (CD4 394/uL) presented with jaundice and acute HBV infection. He was maintained on Cabenuva and monitored regularly. Hepatitis B serologies nine months prior were negative, and liver enzymes were normal. Labs now showed aspartate aminotransferase (AST) 2904 U/L, alanine aminotransferase (ALT) 3995 U/L, bilirubin 11.2 mg/dL, INR 1.1, and acute HBV infection (HBsAg and HBeAg positive, HBV DNA 42.6 million IU/mL). Cabenuva was held due to marked hepatocellular liver injury. Despite modest improvements in liver enzyme levels and HBV viral load (560 IU/mL), bilirubin levels worsened with synthetic dysfunction, anorexia, and weight loss. No hepatic mass or biliary dilation was seen on imaging. Given clinical worsening, Biktarvy, effective against both infections, was started.
Additional testing showed a ferritin level of 6,021 ng/ml with negative serologies for Hepatitis D, E, EBV, and CMV. A liver biopsy revealed acute hepatitis with cholestasis, parenchymal collapse, and stage 4 fibrosis. (Figure 1) CT revealed developing cirrhosis and ascites. Upper endoscopy revealed large, non-bleeding esophageal varices treated with band ligation. Despite HBV suppression, hepatic inflammation persisted, resulting in fibrosis and new-onset portal hypertension. A corticosteroid taper trial showed no benefit. The findings suggest severe hepatotoxicity due to Cabenuva, compounded by acute HBV infection.
Discussion: Cabenuva is well tolerated, with a few cases of mild hepatotoxicity documented. This patient had severe liver injury despite HBV infection control, suggesting the synergistic effect of acute HBV and drug toxicity. The extended half-lives of cabotegravir and rilpivirine (~40 days and 38–43 days, respectively) with measurable levels up to a year post-discontinuation increase the risk of continued liver injury. HBV screening and vaccination are recommended before initiating long-acting ART. Given the increased risk of severe hepatotoxicity with Cabenuva, particularly in the context of acute HBV infection, the need for long-term safety studies for injectable ART is paramount.

Figure: Figure 1. Lab trends correlating with clinical events; CT abdomen and liver histology showing changes consistent with cirrhosis.
Disclosures:
Keri-Ann Buchanan-Peart indicated no relevant financial relationships.
Soe Arker indicated no relevant financial relationships.
Patricia Jones indicated no relevant financial relationships.
Keri-Ann Buchanan-Peart, MD1, Soe H. Arker, MD2, Patricia D. Jones, MD, MSCR3. P1738 - Risk of Severe Hepatotoxicity With Long-Acting Antiretroviral Therapy and Concomitant Acute Hepatitis B Infection: A Rare Clinical Observation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
