Sunday Poster Session
Category: Liver
P1736 - Hepatic and Gastrointestinal Complications in Acute Intermittent Porphyria: Investigating the Role of Iron Metabolism and Disease Progression Factors
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

John Coleman, MD
University of Miami Miller School of Medicine at Holy Cross Hospital
Fort Lauderdale, FL
Presenting Author(s)
John Coleman, MD1, Katelyn Kaufman, DO1, Ingrid C.. Landfald, MD2, Nemer Dabage-Forzoli, MD1, Christopher Lankford, MD3
1University of Miami Miller School of Medicine at Holy Cross Hospital, Fort Lauderdale, FL; 2Department Of Clinical Anatomy Mazovian Academy, Płock, Poland, Poland, Mazowieckie, Poland; 3University of Miami Miller School of Medicine at Holy Cross Hospital, Davie, FL
Introduction: Acute intermittent porphyria (AIP) is a rare autosomal dominant disorder characterized by a deficiency of porphobilinogen deaminase (PBGD), leading to the accumulation of neurotoxic porphyrin precursors. While AIP is primarily considered a neurovisceral disease, emerging evidence suggests an association with hepatic dysfunction, including cirrhosis and hepatocellular carcinoma (HCC).
Case Description/
Methods: We present a 63-year-old male with a history of AIP, cryptogenic liver cirrhosis, and H63D mutation carrier status, who was admitted for recurrent gastrointestinal (GI) bleeding. Serial esophagogastroduodenoscopies (EGDs) demonstrated a progressive worsening of vascular lesions, including gastric antral vascular ectasia (GAVE) and duodenal angioectasias, despite stable cirrhosis. The patient underwent endoscopic interventions, including argon plasma coagulation (APC) and clip placement, with successful hemostasis. The progressive nature of the patient's GI vascular abnormalities raises questions regarding the interplay between AIP, chronic oxidative stress, hepatic dysfunction, and vascular fragility.
Discussion: While portal hypertension remains a primary contributor to GI bleeding in cirrhosis, additional mechanisms such as endothelial dysfunction and porphyrin-related oxidative damage may play a role in vascular ectasia development. Furthermore, the patient’s H63D mutation status, though typically a low-penetrance variant, could contribute to hepatic dysfunction in the presence of other risk factors. This case underscores the need for heightened surveillance in patients with AIP and cirrhosis, particularly regarding progressive vascular pathology and GI bleeding. Future research should investigate the pathophysiologic mechanisms linking AIP to hepatic and vascular complications to optimize long-term management strategies.
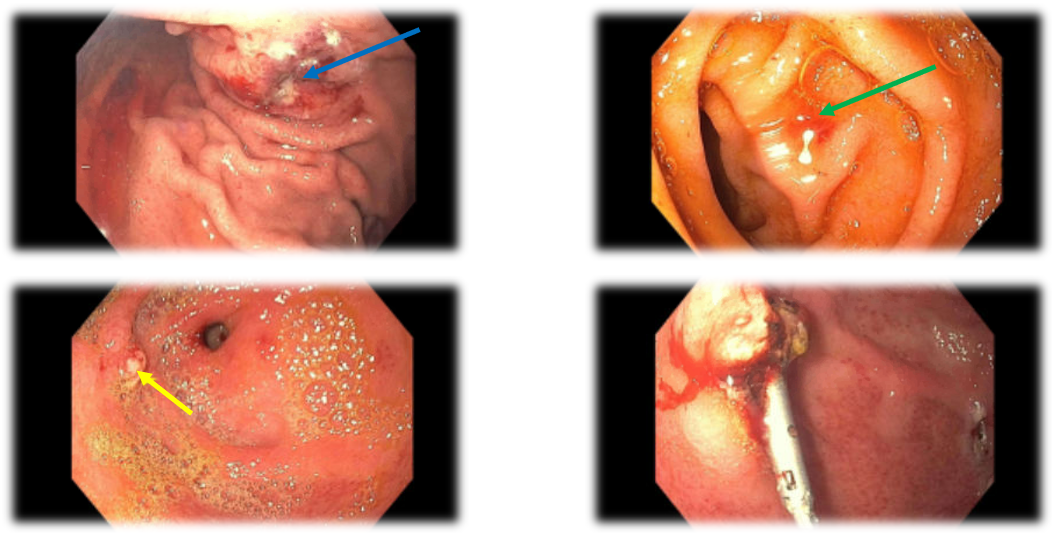
Figure: Figure 1. Images from the patient’s most current esophagogastroduodenoscopy (EGD) procedure showing a gastric antral vascular ectasia with active bleeding (top left, blue arrow) prior to argon plasma coagulation (APC). 2nd portion of the duodenum with recently bleeding angioectasias (top right, green arrow). Imaging of actively bleeding gastric ulcer with visible vessel (bottom left, yellow arrow) and same ulcer status-post successful endoscopic clipping (bottom right).
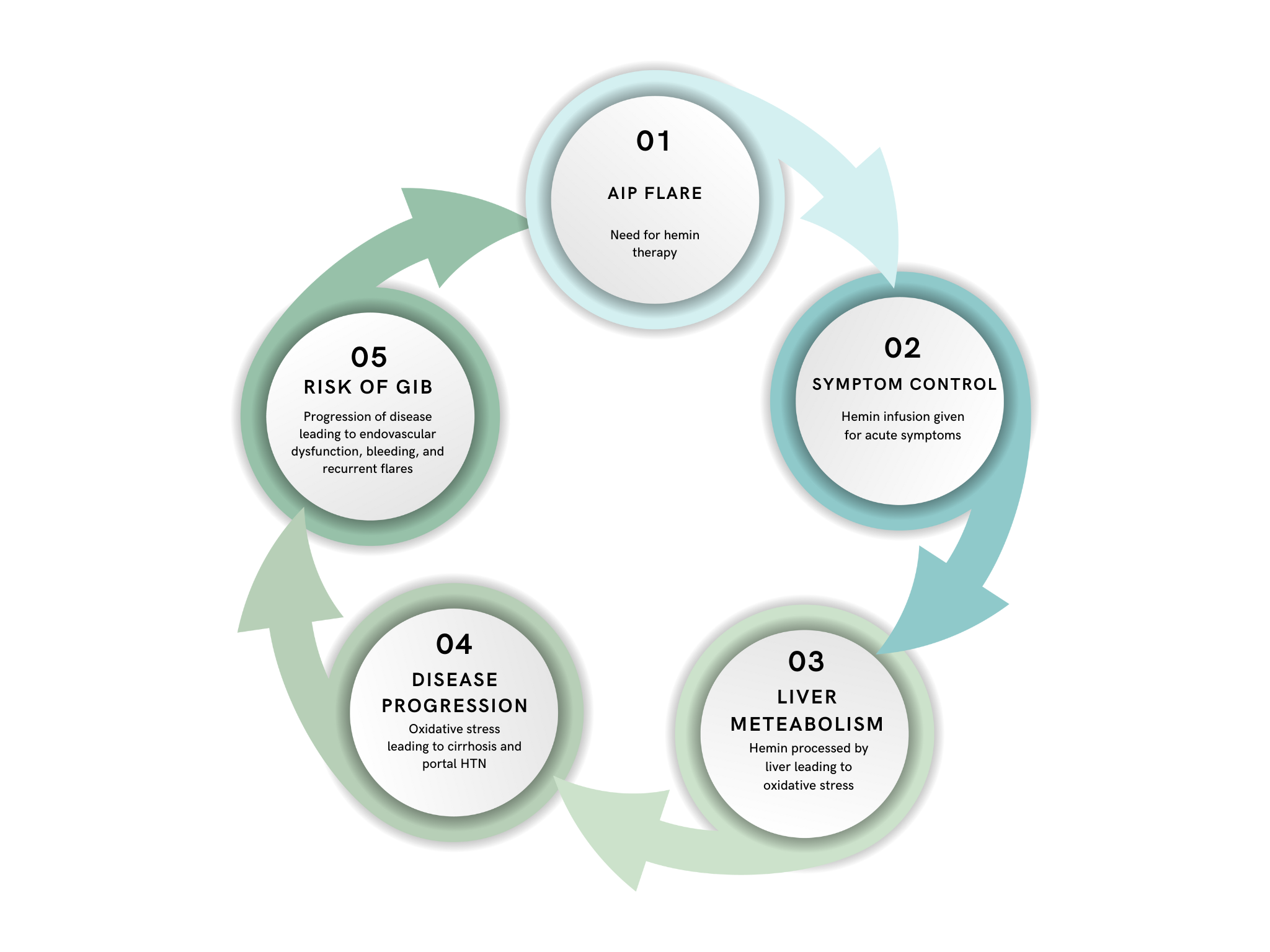
Figure: Diagram detailing possible cycle of an AIP flare resulting in treatment, increased liver metabolism which can lead to progression of the disease and increased risk of bleeding
Disclosures:
John Coleman indicated no relevant financial relationships.
Katelyn Kaufman indicated no relevant financial relationships.
Ingrid Landfald indicated no relevant financial relationships.
Nemer Dabage-Forzoli indicated no relevant financial relationships.
Christopher Lankford indicated no relevant financial relationships.
John Coleman, MD1, Katelyn Kaufman, DO1, Ingrid C.. Landfald, MD2, Nemer Dabage-Forzoli, MD1, Christopher Lankford, MD3. P1736 - Hepatic and Gastrointestinal Complications in Acute Intermittent Porphyria: Investigating the Role of Iron Metabolism and Disease Progression Factors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Miami Miller School of Medicine at Holy Cross Hospital, Fort Lauderdale, FL; 2Department Of Clinical Anatomy Mazovian Academy, Płock, Poland, Poland, Mazowieckie, Poland; 3University of Miami Miller School of Medicine at Holy Cross Hospital, Davie, FL
Introduction: Acute intermittent porphyria (AIP) is a rare autosomal dominant disorder characterized by a deficiency of porphobilinogen deaminase (PBGD), leading to the accumulation of neurotoxic porphyrin precursors. While AIP is primarily considered a neurovisceral disease, emerging evidence suggests an association with hepatic dysfunction, including cirrhosis and hepatocellular carcinoma (HCC).
Case Description/
Methods: We present a 63-year-old male with a history of AIP, cryptogenic liver cirrhosis, and H63D mutation carrier status, who was admitted for recurrent gastrointestinal (GI) bleeding. Serial esophagogastroduodenoscopies (EGDs) demonstrated a progressive worsening of vascular lesions, including gastric antral vascular ectasia (GAVE) and duodenal angioectasias, despite stable cirrhosis. The patient underwent endoscopic interventions, including argon plasma coagulation (APC) and clip placement, with successful hemostasis. The progressive nature of the patient's GI vascular abnormalities raises questions regarding the interplay between AIP, chronic oxidative stress, hepatic dysfunction, and vascular fragility.
Discussion: While portal hypertension remains a primary contributor to GI bleeding in cirrhosis, additional mechanisms such as endothelial dysfunction and porphyrin-related oxidative damage may play a role in vascular ectasia development. Furthermore, the patient’s H63D mutation status, though typically a low-penetrance variant, could contribute to hepatic dysfunction in the presence of other risk factors. This case underscores the need for heightened surveillance in patients with AIP and cirrhosis, particularly regarding progressive vascular pathology and GI bleeding. Future research should investigate the pathophysiologic mechanisms linking AIP to hepatic and vascular complications to optimize long-term management strategies.

Figure: Figure 1. Images from the patient’s most current esophagogastroduodenoscopy (EGD) procedure showing a gastric antral vascular ectasia with active bleeding (top left, blue arrow) prior to argon plasma coagulation (APC). 2nd portion of the duodenum with recently bleeding angioectasias (top right, green arrow). Imaging of actively bleeding gastric ulcer with visible vessel (bottom left, yellow arrow) and same ulcer status-post successful endoscopic clipping (bottom right).

Figure: Diagram detailing possible cycle of an AIP flare resulting in treatment, increased liver metabolism which can lead to progression of the disease and increased risk of bleeding
Disclosures:
John Coleman indicated no relevant financial relationships.
Katelyn Kaufman indicated no relevant financial relationships.
Ingrid Landfald indicated no relevant financial relationships.
Nemer Dabage-Forzoli indicated no relevant financial relationships.
Christopher Lankford indicated no relevant financial relationships.
John Coleman, MD1, Katelyn Kaufman, DO1, Ingrid C.. Landfald, MD2, Nemer Dabage-Forzoli, MD1, Christopher Lankford, MD3. P1736 - Hepatic and Gastrointestinal Complications in Acute Intermittent Porphyria: Investigating the Role of Iron Metabolism and Disease Progression Factors, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
