Sunday Poster Session
Category: Liver
P1680 - Efficacy and Safety of Atezolizumab Plus Bevacizumab Compared to Tyrosine Kinase Inhibitors (Sorafenib and Lenvatinib) as First-Line Therapy for Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- AP
Ananya Prasad (she/her/hers)
ramaiah medical college
Bangalore, Karnataka, India
Presenting Author(s)
Rishikesh R. Magaji, 1, Vinay Chandramouli Bellur, 2, Omar Oudit, DO3, Trisha Chandra Mohan, 1, Ananya Prasad, 4, Vibhav MS, 5, Shradha Chervittara Karaveetil, 4, Deepak Bhat, MBBS6, Keerthi Balaji Babu Naidu, 2, Pavan Kumara Kasam Shiva, 5, Vardhini Ganesh Iyer, 1, Aditya Singh, 7, Allama Prabhu N S, 8, Adithya Sathya narayana, MBBS9
1BGS Global Institute of Medical Sciences, Bangalore, Karnataka, India; 2Ramaiah medical college, Bangalore, Karnataka, India; 3Brookdale University Hospital Medical Center, Brooklyn, NY; 4ramaiah medical college, Bangalore, Karnataka, India; 5bangalore medical college and research institute, Bangalore, Karnataka, India; 6M. S Ramaiah Medical College, Bangalore North, Karnataka, India; 7Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India; 8Bangalore Medical College and Research Institute, Bangalore, Karnataka, India; 9M S Ramaiah Medical College, Bangalore, Karnataka, India
Introduction: The emergence of immunotherapy-based combinations has led to newer First-line treatment options for unresectable hepatocellular carcinoma. This study aims to compare the Efficacy and Safety of Atezolizumab plus Bevacizumab(ATEZO/BEV) versus Sorafenib or Lenvatinib(SOR/LEV), by evaluating the outcomes such as overall survival, progression-free survival and adverse events, to determine the optimal therapeutic strategy for patients with advanced HCC.
Methods: A systematic search was conducted using PubMed, Google Scholar, and Scopus. PRISMA guidelines were followed. A Boolean expression was constructed to retrieve and select articles from major medical databases. Articles that involved analysis of overall survival and progression-free survival following ATEZO/BEV and SOR/LEV therapy for unresectable hepatocellular carcinoma were included in the study.
The difference in mean duration of OS and PFS/DFS following different chemotherapeutic interventions was assessed through the Inverse variance method in R Studio. The Odds ratio of adverse events was assessed using the Mantel-Haenszel method (common effect model), and the Inverse variance method (random effects model) was utilised to analyse the odds ratio. The I^2 test was used to assess the heterogeneity.
Results: The study included a total of 8 articles with 2459 subjects receiving ATEZO/BEV therapy and 2575 subjects receiving SOR/LEV therapy. The PFS/DFS duration was significantly higher in the ATEZO/BEV group as compared to the SOR/LEV group, indicating better clinical efficacy(Mean difference of PFS = 2.58(1.58; 3.60), I^2 = 99%, p < 0.01). The overall survival duration(MDS =1.5834 [-1.8846; 5.0515] I^2 = 100% p=0.3709)and the risk of major adverse events did not change significantly between the two groups.
Discussion: In patients with Unresectable HCC,atezolizumab combined with bevacizumab resulted in better progression-free survival outcomes than sorafenib/lenvatinib. The overall survival duration and adverse events were similar in both groups, indicating a similar safety profile. Similar clinical efficacy and safety end points make both the drug groups viable as first-line options in the treatment of Unresectable HCC. Further trials should focus on assessing safety end points and hepatotoxicity.
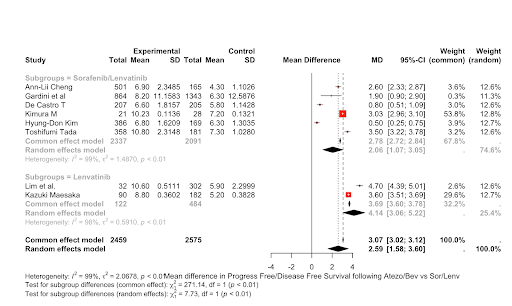
Figure: Mean difference in Progress Free/Disease Free Survival following Atezo/Bev vs Sor/Lenv

Figure: 1.Mean difference in Overall Survival following Atezo/Bev vs Sor/Lenv;
2.Adverse events following Atezo/Bev vs Sor/Lenv therapy
Disclosures:
Rishikesh R. Magaji indicated no relevant financial relationships.
Vinay Chandramouli Bellur indicated no relevant financial relationships.
Omar Oudit indicated no relevant financial relationships.
Trisha Chandra Mohan indicated no relevant financial relationships.
Ananya Prasad indicated no relevant financial relationships.
Vibhav MS indicated no relevant financial relationships.
Shradha Chervittara Karaveetil indicated no relevant financial relationships.
Deepak Bhat indicated no relevant financial relationships.
Keerthi Balaji Babu Naidu indicated no relevant financial relationships.
Pavan Kumara Kasam Shiva indicated no relevant financial relationships.
Vardhini Ganesh Iyer indicated no relevant financial relationships.
Aditya Singh indicated no relevant financial relationships.
Allama Prabhu N S indicated no relevant financial relationships.
Adithya Sathya narayana indicated no relevant financial relationships.
Rishikesh R. Magaji, 1, Vinay Chandramouli Bellur, 2, Omar Oudit, DO3, Trisha Chandra Mohan, 1, Ananya Prasad, 4, Vibhav MS, 5, Shradha Chervittara Karaveetil, 4, Deepak Bhat, MBBS6, Keerthi Balaji Babu Naidu, 2, Pavan Kumara Kasam Shiva, 5, Vardhini Ganesh Iyer, 1, Aditya Singh, 7, Allama Prabhu N S, 8, Adithya Sathya narayana, MBBS9. P1680 - Efficacy and Safety of Atezolizumab Plus Bevacizumab Compared to Tyrosine Kinase Inhibitors (Sorafenib and Lenvatinib) as First-Line Therapy for Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1BGS Global Institute of Medical Sciences, Bangalore, Karnataka, India; 2Ramaiah medical college, Bangalore, Karnataka, India; 3Brookdale University Hospital Medical Center, Brooklyn, NY; 4ramaiah medical college, Bangalore, Karnataka, India; 5bangalore medical college and research institute, Bangalore, Karnataka, India; 6M. S Ramaiah Medical College, Bangalore North, Karnataka, India; 7Bangalore Medical College and Research Institute, Bengaluru, Karnataka, India; 8Bangalore Medical College and Research Institute, Bangalore, Karnataka, India; 9M S Ramaiah Medical College, Bangalore, Karnataka, India
Introduction: The emergence of immunotherapy-based combinations has led to newer First-line treatment options for unresectable hepatocellular carcinoma. This study aims to compare the Efficacy and Safety of Atezolizumab plus Bevacizumab(ATEZO/BEV) versus Sorafenib or Lenvatinib(SOR/LEV), by evaluating the outcomes such as overall survival, progression-free survival and adverse events, to determine the optimal therapeutic strategy for patients with advanced HCC.
Methods: A systematic search was conducted using PubMed, Google Scholar, and Scopus. PRISMA guidelines were followed. A Boolean expression was constructed to retrieve and select articles from major medical databases. Articles that involved analysis of overall survival and progression-free survival following ATEZO/BEV and SOR/LEV therapy for unresectable hepatocellular carcinoma were included in the study.
The difference in mean duration of OS and PFS/DFS following different chemotherapeutic interventions was assessed through the Inverse variance method in R Studio. The Odds ratio of adverse events was assessed using the Mantel-Haenszel method (common effect model), and the Inverse variance method (random effects model) was utilised to analyse the odds ratio. The I^2 test was used to assess the heterogeneity.
Results: The study included a total of 8 articles with 2459 subjects receiving ATEZO/BEV therapy and 2575 subjects receiving SOR/LEV therapy. The PFS/DFS duration was significantly higher in the ATEZO/BEV group as compared to the SOR/LEV group, indicating better clinical efficacy(Mean difference of PFS = 2.58(1.58; 3.60), I^2 = 99%, p < 0.01). The overall survival duration(MDS =1.5834 [-1.8846; 5.0515] I^2 = 100% p=0.3709)and the risk of major adverse events did not change significantly between the two groups.
Discussion: In patients with Unresectable HCC,atezolizumab combined with bevacizumab resulted in better progression-free survival outcomes than sorafenib/lenvatinib. The overall survival duration and adverse events were similar in both groups, indicating a similar safety profile. Similar clinical efficacy and safety end points make both the drug groups viable as first-line options in the treatment of Unresectable HCC. Further trials should focus on assessing safety end points and hepatotoxicity.

Figure: Mean difference in Progress Free/Disease Free Survival following Atezo/Bev vs Sor/Lenv

Figure: 1.Mean difference in Overall Survival following Atezo/Bev vs Sor/Lenv;
2.Adverse events following Atezo/Bev vs Sor/Lenv therapy
Disclosures:
Rishikesh R. Magaji indicated no relevant financial relationships.
Vinay Chandramouli Bellur indicated no relevant financial relationships.
Omar Oudit indicated no relevant financial relationships.
Trisha Chandra Mohan indicated no relevant financial relationships.
Ananya Prasad indicated no relevant financial relationships.
Vibhav MS indicated no relevant financial relationships.
Shradha Chervittara Karaveetil indicated no relevant financial relationships.
Deepak Bhat indicated no relevant financial relationships.
Keerthi Balaji Babu Naidu indicated no relevant financial relationships.
Pavan Kumara Kasam Shiva indicated no relevant financial relationships.
Vardhini Ganesh Iyer indicated no relevant financial relationships.
Aditya Singh indicated no relevant financial relationships.
Allama Prabhu N S indicated no relevant financial relationships.
Adithya Sathya narayana indicated no relevant financial relationships.
Rishikesh R. Magaji, 1, Vinay Chandramouli Bellur, 2, Omar Oudit, DO3, Trisha Chandra Mohan, 1, Ananya Prasad, 4, Vibhav MS, 5, Shradha Chervittara Karaveetil, 4, Deepak Bhat, MBBS6, Keerthi Balaji Babu Naidu, 2, Pavan Kumara Kasam Shiva, 5, Vardhini Ganesh Iyer, 1, Aditya Singh, 7, Allama Prabhu N S, 8, Adithya Sathya narayana, MBBS9. P1680 - Efficacy and Safety of Atezolizumab Plus Bevacizumab Compared to Tyrosine Kinase Inhibitors (Sorafenib and Lenvatinib) as First-Line Therapy for Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
