Sunday Poster Session
Category: Liver
P1627 - Heterogeneity in Randomized Controlled Trials of Corticosteroid Use for Alcoholic Hepatitis: Implications for Efficacy and Infection Risk
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Rithish Nimmagadda, MBBS (he/him/his)
One Brooklyn Health-Interfaith Medical Center
Brooklyn, NY
Presenting Author(s)
Rithish Nimmagadda, MBBS1, Naga Sai Akhil Reddy Gogula, MBBS2, Pragathi Munnangi, MD3, Kesava Manikanta Achuta, MD4, Nayanika Tummala, MD5, Naga Praneeth Vakkalagadda, MD6, Sameer Majety, MBBS7, Rahul Khandekar, MD8, Sai Prasad Karuturi, MBBS9
1One Brooklyn Health-Interfaith Medical Center, Brooklyn, NY; 2Duke University, Durham, NC; 3BronxCare Health System, New York City, NY; 4Garden City Hospital, Garden City, MI; 5St. Mary's General Hospital, New York Medical College, Poughkeepsie, NY; 6University of Alabama at Birmingham Heersink School of Medicine, Montgomery, AL; 7School of Medicine, Xiamen University., Kakinada, Andhra Pradesh, India; 8NYU Grossman School of Medicine, Brooklyn, NY; 9SVS medical college, Hyderabad, Telangana, India
Introduction: Corticosteroids are the mainstay treatment for severe alcoholic hepatitis (AH), yet randomized controlled trials (RCTs) report conflicting outcomes. A key contributor to this is heterogeneity in study design, including differences in steroid regimens, patient selection, infection surveillance, and follow-up. This systematic review evaluates RCTs of corticosteroids in AH to assess heterogeneity in study design and outcomes reporting.
Methods: Twenty-Nine RCTs evaluating corticosteroid therapy in AH were analyzed. Key variables included steroid regimen, tapering protocols, treatment duration, initiation timing, comparator arms, geographic setting, and infection outcomes. Infections were categorized as bacterial (B), fungal (F), viral (V), or not specified (NS), and reported at baseline and during follow-up. Studies were also classified by the presence and duration of follow-up protocols.
Results: Trial designs showed substantial heterogeneity. Steroid regimens varied from prednisolone 40 mg daily to pulse methylprednisolone or tapered protocols. Tapering strategies ranged from none to 12-day, 2-week, or 4-week regimens. Timing of initiation spanned from < 3 to >13 days post-admission. Comparators included placebo, pentoxifylline, nutritional therapy, antibiotics, immunomodulators, and antioxidants. Infection outcomes were inconsistently reported. Of 18 studies with available data, initial bacterial infections were reported in 46% (Louvet et al) and 24% (Phillips et al). Follow-up infections were common, with bacterial infections most frequent, followed by fungal and viral types. Follow-up duration ranged from 6 days to 12 months, limiting comparability of infection-related outcomes.
Discussion: Significant heterogeneity in RCT design for corticosteroid use in alcoholic hepatitis particularly in dosing, initiation timing, tapering, and control arms hinders cross-trial comparisons and meta-analytic interpretation. Infections, a common and prognostically important complication, are inconsistently reported and variably defined. Standardized infection surveillance, uniform follow-up durations, and stratified reporting by infection type and timing are needed in future trials to better define the risk-benefit profile of corticosteroids in AH and guide clinical management.
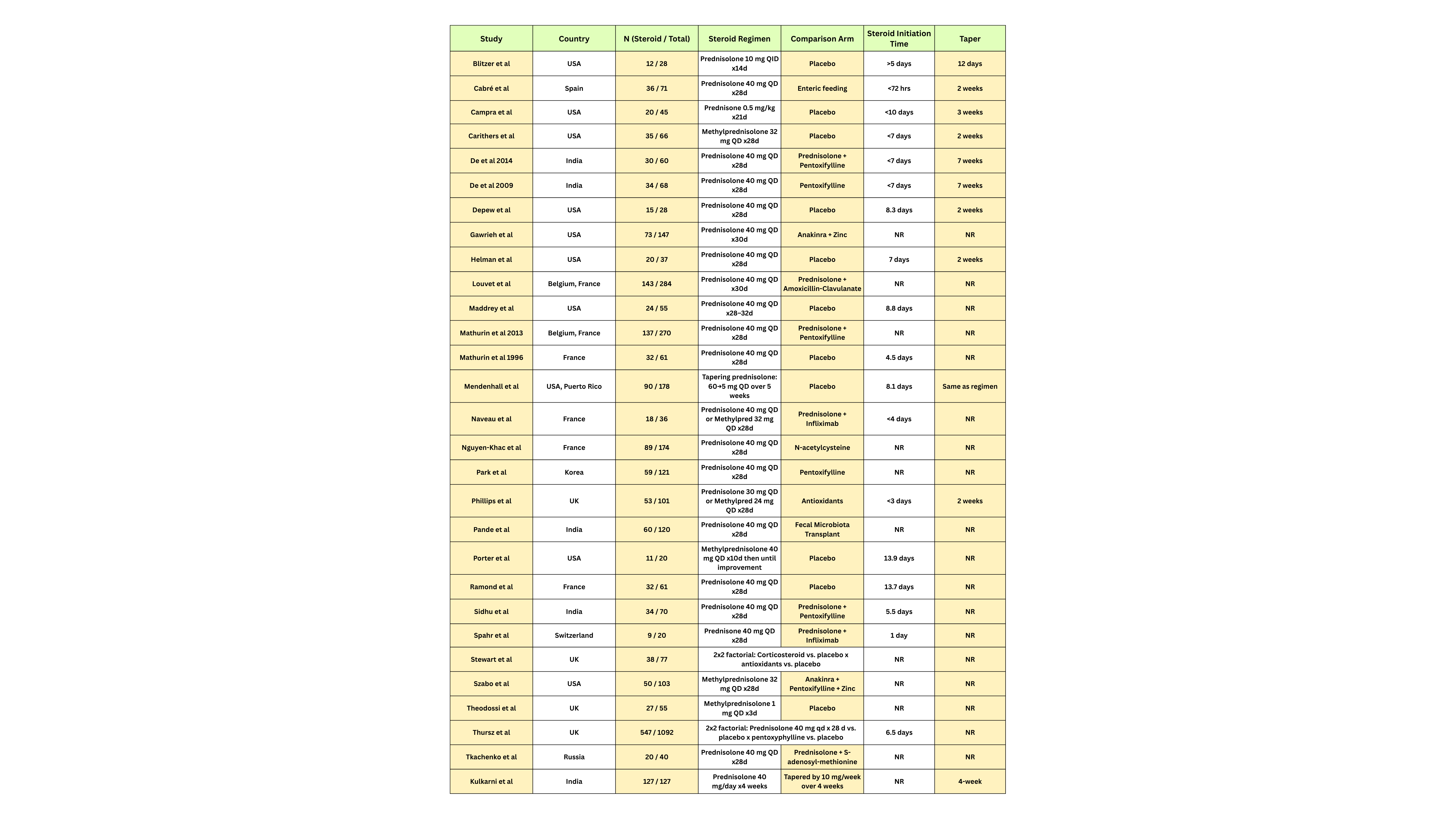
Figure: Table 1 - Summary of Randomized Controlled Trials Assessing Steroid Use in Alcohol-Associated Hepatitis: Regimens, Comparators, and Tapering Strategies
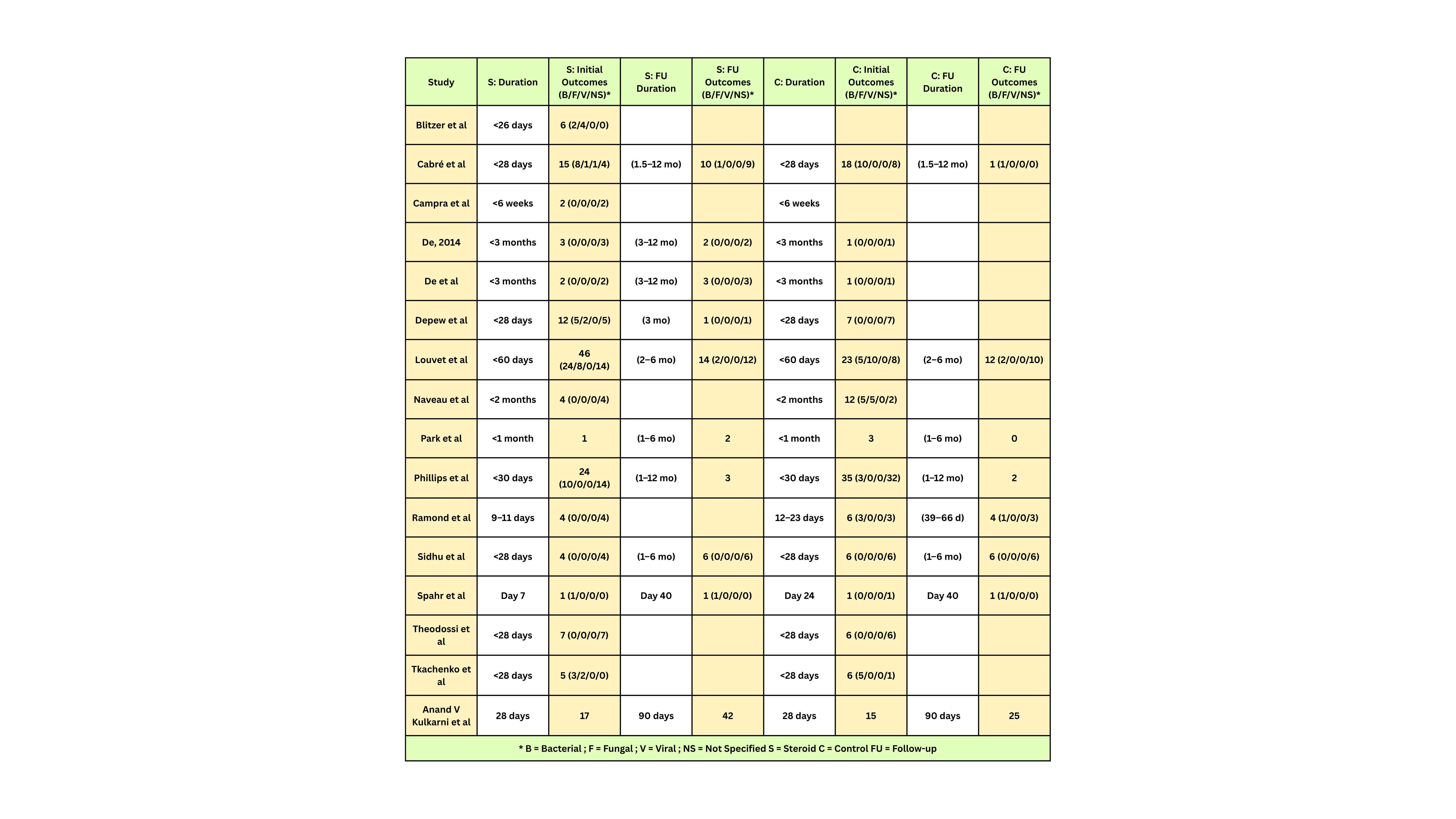
Figure: Table 2- Infectious Complications in Steroid vs. Control Arms Across RCTs for Alcohol-Associated Hepatitis: Timing, Type, and Follow-Up
Disclosures:
Rithish Nimmagadda indicated no relevant financial relationships.
Naga Sai Akhil Reddy Gogula indicated no relevant financial relationships.
Pragathi Munnangi indicated no relevant financial relationships.
Kesava Manikanta Achuta indicated no relevant financial relationships.
Nayanika Tummala indicated no relevant financial relationships.
Naga Praneeth Vakkalagadda indicated no relevant financial relationships.
Sameer Majety indicated no relevant financial relationships.
Rahul Khandekar indicated no relevant financial relationships.
Sai Prasad Karuturi indicated no relevant financial relationships.
Rithish Nimmagadda, MBBS1, Naga Sai Akhil Reddy Gogula, MBBS2, Pragathi Munnangi, MD3, Kesava Manikanta Achuta, MD4, Nayanika Tummala, MD5, Naga Praneeth Vakkalagadda, MD6, Sameer Majety, MBBS7, Rahul Khandekar, MD8, Sai Prasad Karuturi, MBBS9. P1627 - Heterogeneity in Randomized Controlled Trials of Corticosteroid Use for Alcoholic Hepatitis: Implications for Efficacy and Infection Risk, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1One Brooklyn Health-Interfaith Medical Center, Brooklyn, NY; 2Duke University, Durham, NC; 3BronxCare Health System, New York City, NY; 4Garden City Hospital, Garden City, MI; 5St. Mary's General Hospital, New York Medical College, Poughkeepsie, NY; 6University of Alabama at Birmingham Heersink School of Medicine, Montgomery, AL; 7School of Medicine, Xiamen University., Kakinada, Andhra Pradesh, India; 8NYU Grossman School of Medicine, Brooklyn, NY; 9SVS medical college, Hyderabad, Telangana, India
Introduction: Corticosteroids are the mainstay treatment for severe alcoholic hepatitis (AH), yet randomized controlled trials (RCTs) report conflicting outcomes. A key contributor to this is heterogeneity in study design, including differences in steroid regimens, patient selection, infection surveillance, and follow-up. This systematic review evaluates RCTs of corticosteroids in AH to assess heterogeneity in study design and outcomes reporting.
Methods: Twenty-Nine RCTs evaluating corticosteroid therapy in AH were analyzed. Key variables included steroid regimen, tapering protocols, treatment duration, initiation timing, comparator arms, geographic setting, and infection outcomes. Infections were categorized as bacterial (B), fungal (F), viral (V), or not specified (NS), and reported at baseline and during follow-up. Studies were also classified by the presence and duration of follow-up protocols.
Results: Trial designs showed substantial heterogeneity. Steroid regimens varied from prednisolone 40 mg daily to pulse methylprednisolone or tapered protocols. Tapering strategies ranged from none to 12-day, 2-week, or 4-week regimens. Timing of initiation spanned from < 3 to >13 days post-admission. Comparators included placebo, pentoxifylline, nutritional therapy, antibiotics, immunomodulators, and antioxidants. Infection outcomes were inconsistently reported. Of 18 studies with available data, initial bacterial infections were reported in 46% (Louvet et al) and 24% (Phillips et al). Follow-up infections were common, with bacterial infections most frequent, followed by fungal and viral types. Follow-up duration ranged from 6 days to 12 months, limiting comparability of infection-related outcomes.
Discussion: Significant heterogeneity in RCT design for corticosteroid use in alcoholic hepatitis particularly in dosing, initiation timing, tapering, and control arms hinders cross-trial comparisons and meta-analytic interpretation. Infections, a common and prognostically important complication, are inconsistently reported and variably defined. Standardized infection surveillance, uniform follow-up durations, and stratified reporting by infection type and timing are needed in future trials to better define the risk-benefit profile of corticosteroids in AH and guide clinical management.

Figure: Table 1 - Summary of Randomized Controlled Trials Assessing Steroid Use in Alcohol-Associated Hepatitis: Regimens, Comparators, and Tapering Strategies

Figure: Table 2- Infectious Complications in Steroid vs. Control Arms Across RCTs for Alcohol-Associated Hepatitis: Timing, Type, and Follow-Up
Disclosures:
Rithish Nimmagadda indicated no relevant financial relationships.
Naga Sai Akhil Reddy Gogula indicated no relevant financial relationships.
Pragathi Munnangi indicated no relevant financial relationships.
Kesava Manikanta Achuta indicated no relevant financial relationships.
Nayanika Tummala indicated no relevant financial relationships.
Naga Praneeth Vakkalagadda indicated no relevant financial relationships.
Sameer Majety indicated no relevant financial relationships.
Rahul Khandekar indicated no relevant financial relationships.
Sai Prasad Karuturi indicated no relevant financial relationships.
Rithish Nimmagadda, MBBS1, Naga Sai Akhil Reddy Gogula, MBBS2, Pragathi Munnangi, MD3, Kesava Manikanta Achuta, MD4, Nayanika Tummala, MD5, Naga Praneeth Vakkalagadda, MD6, Sameer Majety, MBBS7, Rahul Khandekar, MD8, Sai Prasad Karuturi, MBBS9. P1627 - Heterogeneity in Randomized Controlled Trials of Corticosteroid Use for Alcoholic Hepatitis: Implications for Efficacy and Infection Risk, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
