Sunday Poster Session
Category: Liver
P1624 - Limited Approvals in MASLD Highlight the Need to Reevaluate FDA Guidance for Outcome Measures
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Akanksha Togra, MD (she/her/hers)
Texas Tech University Health Sciences Center, El Paso
El Paso, TX
Presenting Author(s)
Akanksha Togra, MD1, Adderly Toribio de Jesus, MD2, Indraneel Abhijit. Deshmukh, MBBS3, Shefali Mody, MBBS4, Silpa Choday, MD5, Sushrut Ingawale, MD, DNB, MBBS6, Alejandro Robles, MD7
1Texas Tech University Health Sciences Center, El Paso, El Paso, TX; 2Division of Gastroenterology, Department of Internal Medicine, Texas Tech University Health Sciences Center, El Paso, TX., El Paso, TX; 3MD Anderson Cancer Center, Houston, TX; 4SUNY Upstate Medical University Hospital, Syracuse, NY; 5Creighton University School of Medicine, Phoenix, AZ; 6Quinnipiac University - Frank H Netter MD School of Medicine, Bridgeport, CT; 7Department of Gastroenterology, Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center El Paso, El Paso , TX, El Paso, TX
Introduction: Metabolic dysfunction associated fatty liver disease (MASLD) affects about 24% US adult population. Despite high burden, off label medications such as GLP-1 receptor agonists and Vitamin E continue to be the mainstay of guideline recommended therapy for MASLD. Our study analyzes randomized controlled trials (RCTs) in MASLD, against FDA-defined outcome measures, to evaluate their efficacy and the potential need for revising current regulatory guidance.
Methods: Data was extracted from PubMed, Medline and Cochrane library. Outcome measures were defined per FDA guidance: 1) Resolution of steatohepatitis without worsening of liver fibrosis 2) Improvement in liver fibrosis greater than or equal to one stage and no worsening of steatohepatitis 3) both.
Results: Of the 464 RCTs identified, only 63 were assessing pharmacotherapies. Despite these drugs demonstrating meaningful improvement through non-invasive assessments such as FibroScan and MRI-PDFF, over 75% of these studies were not eligible for FDA approval pathways, given the current regulatory requirement of biopsy-based endpoints. Only 10 of the 63 studies were evaluating liver biopsy based on steatohepatitis resolution (Figure 1) or fibrosis improvement (Figure 2) and included in our analysis. SGLT-2 inhibitor, Ipragliflozin demonstrated significant improvement in fibrosis (OR 7; 95% CI 1.77-27.68), however it's effect on steatohepatitis was not reported. While GLP-1 receptor agonist (GLP-1 RA) Liraglutide had significant impact of steatohepatitis resolution (OR 6.43; 95% CI 1.20-34.41) in a phase 2 trial without a follow up phase 3 study. Resmetirom was the only drug effective in both MASLD/MASH resolution (OR 3.24; 95% CI 2.07-5.08) and fibrosis improvement (OR 1.92; 95% CI 1.28-2.89) as demonstrated in the landmark MAESTRO-NASH trial. Obeticholic acid did show improvement in fibrosis (OR 2.22; 95% CI 1.44-3.42), but did not receive FDA approval due to its risk-benefit profile.
Discussion: Resmetirom is currently the only FDA-approved therapy for MASLD. Ongoing concerns persist regarding the FDA’s stringent endpoints for approval. Although non-invasive tools are widely used in clinical practice, they are not yet accepted for regulatory decision-making. Due to these reasons, GLP-1 RAs and SGLT2 inhibitors remain unapproved despite promising. There is an urgent need to optimize FDA approval end points to expand the treatment landscape and improve care for patients with MASLD.
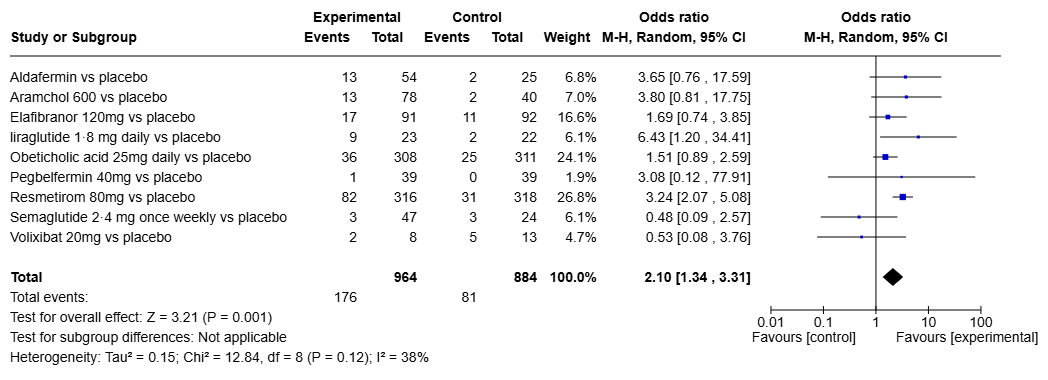
Figure: Figure 1: Outcome measure - MASLD resolution without worsening of fibrosis
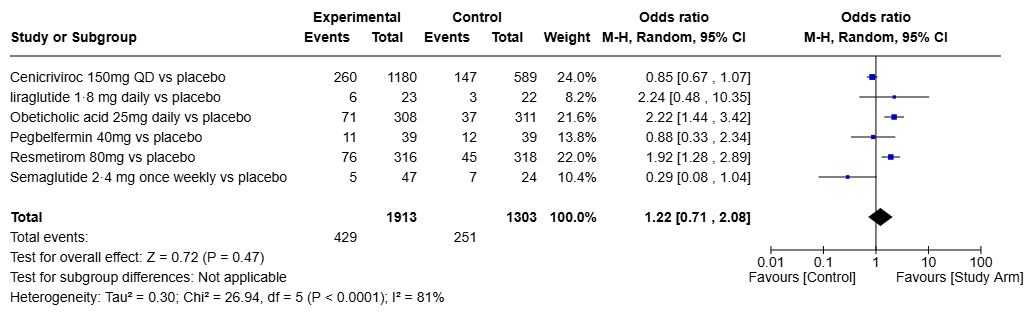
Figure: Figure 2: Outcome measure - Improvement of fibrosis by at least 1 stage without worsening of steatohepatitis
Disclosures:
Akanksha Togra indicated no relevant financial relationships.
Adderly Toribio de Jesus indicated no relevant financial relationships.
Indraneel Deshmukh indicated no relevant financial relationships.
Shefali Mody indicated no relevant financial relationships.
Silpa Choday indicated no relevant financial relationships.
Sushrut Ingawale indicated no relevant financial relationships.
Alejandro Robles indicated no relevant financial relationships.
Akanksha Togra, MD1, Adderly Toribio de Jesus, MD2, Indraneel Abhijit. Deshmukh, MBBS3, Shefali Mody, MBBS4, Silpa Choday, MD5, Sushrut Ingawale, MD, DNB, MBBS6, Alejandro Robles, MD7. P1624 - Limited Approvals in MASLD Highlight the Need to Reevaluate FDA Guidance for Outcome Measures, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Texas Tech University Health Sciences Center, El Paso, El Paso, TX; 2Division of Gastroenterology, Department of Internal Medicine, Texas Tech University Health Sciences Center, El Paso, TX., El Paso, TX; 3MD Anderson Cancer Center, Houston, TX; 4SUNY Upstate Medical University Hospital, Syracuse, NY; 5Creighton University School of Medicine, Phoenix, AZ; 6Quinnipiac University - Frank H Netter MD School of Medicine, Bridgeport, CT; 7Department of Gastroenterology, Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center El Paso, El Paso , TX, El Paso, TX
Introduction: Metabolic dysfunction associated fatty liver disease (MASLD) affects about 24% US adult population. Despite high burden, off label medications such as GLP-1 receptor agonists and Vitamin E continue to be the mainstay of guideline recommended therapy for MASLD. Our study analyzes randomized controlled trials (RCTs) in MASLD, against FDA-defined outcome measures, to evaluate their efficacy and the potential need for revising current regulatory guidance.
Methods: Data was extracted from PubMed, Medline and Cochrane library. Outcome measures were defined per FDA guidance: 1) Resolution of steatohepatitis without worsening of liver fibrosis 2) Improvement in liver fibrosis greater than or equal to one stage and no worsening of steatohepatitis 3) both.
Results: Of the 464 RCTs identified, only 63 were assessing pharmacotherapies. Despite these drugs demonstrating meaningful improvement through non-invasive assessments such as FibroScan and MRI-PDFF, over 75% of these studies were not eligible for FDA approval pathways, given the current regulatory requirement of biopsy-based endpoints. Only 10 of the 63 studies were evaluating liver biopsy based on steatohepatitis resolution (Figure 1) or fibrosis improvement (Figure 2) and included in our analysis. SGLT-2 inhibitor, Ipragliflozin demonstrated significant improvement in fibrosis (OR 7; 95% CI 1.77-27.68), however it's effect on steatohepatitis was not reported. While GLP-1 receptor agonist (GLP-1 RA) Liraglutide had significant impact of steatohepatitis resolution (OR 6.43; 95% CI 1.20-34.41) in a phase 2 trial without a follow up phase 3 study. Resmetirom was the only drug effective in both MASLD/MASH resolution (OR 3.24; 95% CI 2.07-5.08) and fibrosis improvement (OR 1.92; 95% CI 1.28-2.89) as demonstrated in the landmark MAESTRO-NASH trial. Obeticholic acid did show improvement in fibrosis (OR 2.22; 95% CI 1.44-3.42), but did not receive FDA approval due to its risk-benefit profile.
Discussion: Resmetirom is currently the only FDA-approved therapy for MASLD. Ongoing concerns persist regarding the FDA’s stringent endpoints for approval. Although non-invasive tools are widely used in clinical practice, they are not yet accepted for regulatory decision-making. Due to these reasons, GLP-1 RAs and SGLT2 inhibitors remain unapproved despite promising. There is an urgent need to optimize FDA approval end points to expand the treatment landscape and improve care for patients with MASLD.

Figure: Figure 1: Outcome measure - MASLD resolution without worsening of fibrosis

Figure: Figure 2: Outcome measure - Improvement of fibrosis by at least 1 stage without worsening of steatohepatitis
Disclosures:
Akanksha Togra indicated no relevant financial relationships.
Adderly Toribio de Jesus indicated no relevant financial relationships.
Indraneel Deshmukh indicated no relevant financial relationships.
Shefali Mody indicated no relevant financial relationships.
Silpa Choday indicated no relevant financial relationships.
Sushrut Ingawale indicated no relevant financial relationships.
Alejandro Robles indicated no relevant financial relationships.
Akanksha Togra, MD1, Adderly Toribio de Jesus, MD2, Indraneel Abhijit. Deshmukh, MBBS3, Shefali Mody, MBBS4, Silpa Choday, MD5, Sushrut Ingawale, MD, DNB, MBBS6, Alejandro Robles, MD7. P1624 - Limited Approvals in MASLD Highlight the Need to Reevaluate FDA Guidance for Outcome Measures, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
