Sunday Poster Session
Category: Liver
P1596 - Hepatitis B Virus Screening Prior to Immunosuppressive Therapy in Veterans With Inflammatory Bowel Disease: A Quality Improvement Opportunity
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Jacky Reny, MD (he/him/his)
Stony Brook University Hospital
Port Jefferson Station, NY
Presenting Author(s)
Jacky Reny, MD1, Jordan Barnett-Kradjian, DO2, Kaustav Patra, MD1, Pallavi Kawatra, BS3, Lisa Fisher, MD4
1Stony Brook University Hospital, Port Jefferson Station, NY; 2Stony Brook Medicine, Stony Brook, NY; 3Philadelphia College of Osteopathic Medicine, Philadelphia, PA; 4Stony Brook University Hospital, Northport, NY
Introduction: Screening for hepatitis B virus (HBV) prior to initiating immunosuppressive therapy is a critical safety practice in the management of patients with inflammatory bowel disease (IBD). Reactivation of latent HBV can result in severe morbidity and mortality, yet adherence to HBV screening guidelines remains variable in clinical practice, particularly among high-risk populations. We aimed to assess the rates and timeliness of HBV screening prior to immunosuppressive therapy in a VA IBD cohort, identify missed opportunities, and propose interventions to improve adherence.
Methods: We conducted a retrospective chart review of Veterans diagnosed with IBD who were newly initiated on immunosuppressive medications (thiopurines, methotrexate, or biologic agents) between 2022-2024 at a single VA medical center. Patients were identified via pharmacy records and ICD codes. The primary outcome was the proportion of patients with complete HBV serology testing—defined as documentation of hepatitis B surface antigen, core antibody, and surface antibody—within 6 months prior to starting immunosuppression. Secondary outcomes included the time interval between screening and therapy initiation, variation by medication class, and factors associated with incomplete screening.
Results: A total of 148 Veterans with IBD were newly started on immunosuppressive therapy during the study period. Only 62% underwent complete HBV screening prior to therapy initiation. Among those screened, the median interval from serology testing to therapy initiation was 19 days (range: 2–120 days). Screening rates were highest among patients prescribed biologics (75%) compared to thiopurines (54%) and methotrexate (51%). Notably, 38% of patients had incomplete or absent HBV testing prior to immunosuppression, representing a significant care gap. No significant differences in screening rates were observed by age, sex, or IBD subtype.
Discussion: HBV screening prior to immunosuppressive therapy in Veterans with IBD is suboptimal, with over one-third of patients lacking complete pre-treatment serology. Biologic therapy was associated with higher screening rates, but overall adherence remains below established guidelines. These findings highlight a critical quality improvement opportunity. Proposed interventions include electronic health record alerts, standardized order sets, and provider education to improve screening rates, reduce preventable HBV reactivation, and enhance patient safety in the VA IBD population.
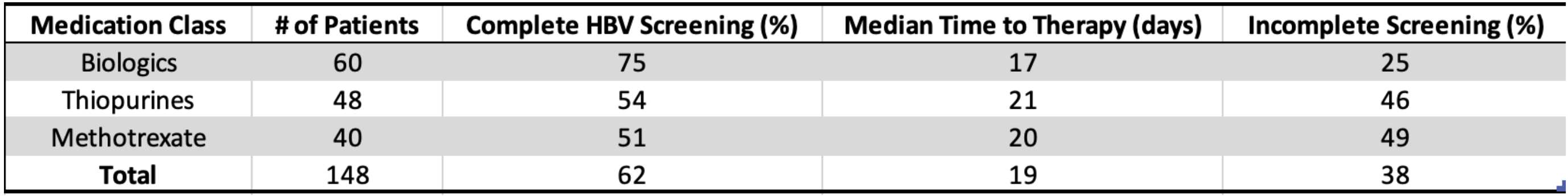
Figure: HBV Screening Completion by Medication Class
Disclosures:
Jacky Reny indicated no relevant financial relationships.
Jordan Barnett-Kradjian indicated no relevant financial relationships.
Kaustav Patra indicated no relevant financial relationships.
Pallavi Kawatra indicated no relevant financial relationships.
Lisa Fisher indicated no relevant financial relationships.
Jacky Reny, MD1, Jordan Barnett-Kradjian, DO2, Kaustav Patra, MD1, Pallavi Kawatra, BS3, Lisa Fisher, MD4. P1596 - Hepatitis B Virus Screening Prior to Immunosuppressive Therapy in Veterans With Inflammatory Bowel Disease: A Quality Improvement Opportunity, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Stony Brook University Hospital, Port Jefferson Station, NY; 2Stony Brook Medicine, Stony Brook, NY; 3Philadelphia College of Osteopathic Medicine, Philadelphia, PA; 4Stony Brook University Hospital, Northport, NY
Introduction: Screening for hepatitis B virus (HBV) prior to initiating immunosuppressive therapy is a critical safety practice in the management of patients with inflammatory bowel disease (IBD). Reactivation of latent HBV can result in severe morbidity and mortality, yet adherence to HBV screening guidelines remains variable in clinical practice, particularly among high-risk populations. We aimed to assess the rates and timeliness of HBV screening prior to immunosuppressive therapy in a VA IBD cohort, identify missed opportunities, and propose interventions to improve adherence.
Methods: We conducted a retrospective chart review of Veterans diagnosed with IBD who were newly initiated on immunosuppressive medications (thiopurines, methotrexate, or biologic agents) between 2022-2024 at a single VA medical center. Patients were identified via pharmacy records and ICD codes. The primary outcome was the proportion of patients with complete HBV serology testing—defined as documentation of hepatitis B surface antigen, core antibody, and surface antibody—within 6 months prior to starting immunosuppression. Secondary outcomes included the time interval between screening and therapy initiation, variation by medication class, and factors associated with incomplete screening.
Results: A total of 148 Veterans with IBD were newly started on immunosuppressive therapy during the study period. Only 62% underwent complete HBV screening prior to therapy initiation. Among those screened, the median interval from serology testing to therapy initiation was 19 days (range: 2–120 days). Screening rates were highest among patients prescribed biologics (75%) compared to thiopurines (54%) and methotrexate (51%). Notably, 38% of patients had incomplete or absent HBV testing prior to immunosuppression, representing a significant care gap. No significant differences in screening rates were observed by age, sex, or IBD subtype.
Discussion: HBV screening prior to immunosuppressive therapy in Veterans with IBD is suboptimal, with over one-third of patients lacking complete pre-treatment serology. Biologic therapy was associated with higher screening rates, but overall adherence remains below established guidelines. These findings highlight a critical quality improvement opportunity. Proposed interventions include electronic health record alerts, standardized order sets, and provider education to improve screening rates, reduce preventable HBV reactivation, and enhance patient safety in the VA IBD population.

Figure: HBV Screening Completion by Medication Class
Disclosures:
Jacky Reny indicated no relevant financial relationships.
Jordan Barnett-Kradjian indicated no relevant financial relationships.
Kaustav Patra indicated no relevant financial relationships.
Pallavi Kawatra indicated no relevant financial relationships.
Lisa Fisher indicated no relevant financial relationships.
Jacky Reny, MD1, Jordan Barnett-Kradjian, DO2, Kaustav Patra, MD1, Pallavi Kawatra, BS3, Lisa Fisher, MD4. P1596 - Hepatitis B Virus Screening Prior to Immunosuppressive Therapy in Veterans With Inflammatory Bowel Disease: A Quality Improvement Opportunity, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
