Sunday Poster Session
Category: Liver
P1565 - Probiotic Lactobacillus Rhamnosus GG Modulates Dietary Tryptophan-Induced Hepatic Reprogramming in Mice
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
.jpg)
Amy Narakornpichit, BS
Rutgers New Jersey Medical School
Newark, NJ
Presenting Author(s)
Award: ACG Presidential Poster Award
Amy Narakornpichit, BS1, Jayson Antonio, PhD1, Josh Kanattu, BS1, A.J. Justice, MBS1, Panan Suntornsaratoon, PhD2, Nan Gao, PhD1, Ronaldo Ferraris, PhD1
1Rutgers New Jersey Medical School, Newark, NJ; 2Rutgers New Jersey Medical School, Salaya, Nakhon Pathom, Thailand
Introduction: Dietary tryptophan (Trp) shapes microbial metabolite production, and its deficiency is strongly associated with the pathogenesis of metabolic dysfunction-associated steatotic liver disease (MASLD), which affects over 100 million Americans. Probiotic bacteria have been shown to mitigate gut dysbiosis and liver inflammation in MASLD, yet how diet and probiotics interact to influence hepatic signaling remains poorly defined. We integrated transcriptomic (~18,500 genes) and metabolomic profiling to investigate how Trp availability and probiotic Lactobacillus rhamnosus GG (LGG) supplementation jointly modulate hepatic signaling networks in mice.
Methods: Germ-free mice were fed a Trp-sufficient (TRP+) or Trp-deficient (TRP-) diet for two weeks, followed by a single oral gavage of LGG or PBS (vehicle), and remained on their diets for three more weeks. Liver, serum, and fecal samples were analyzed by RNA-seq and metabolomics. Differential gene expression was assessed with DESeq2; gene set enrichment analysis identified key pathways; and metabolome-transcriptome correlation analysis (MeTCRA) revealed gene-metabolite associations.
Results: Principal component analysis revealed that Trp availability is the dominant driver of both hepatic metabolomic and transcriptomic profiles. In TRP- mice, LGG had minimal impact on gene expression, underscoring the necessity of Trp for microbiota-driven hepatic regulation. In contrast, under TRP+ conditions, LGG synergistically altered liver metabolites and substantially remodeled hepatic gene expression, activating oxidative phosphorylation, MYC/E2F-mediated proliferation, and mTOR signaling pathways. Although LGG induced only 38 differentially expressed genes in TRP+ mice (TRP+LGG vs TRP+PBS), enriched pathways were broad—spanning proliferation, barrier function, and the unfolded protein response. MeTCRA linked hepatic taurine, tyrosine, and NAD+, to these responses, highlighting the interplay between dietary amino acids, gut microbiota, and energy-regulatory circuits.
Discussion: LGG-driven hepatic effects are highly contingent on Trp sufficiency. Under adequate dietary conditions, LGG initiates a coordinated transcriptional and metabolic cascade involving immune, stress, and proliferative networks. These findings establish a critical diet–microbiome–liver axis and support the development of targeted nutritional and microbial strategies for managing MASLD and related disorders. AI-assisted language editing only; all scientific content developed by the authors.
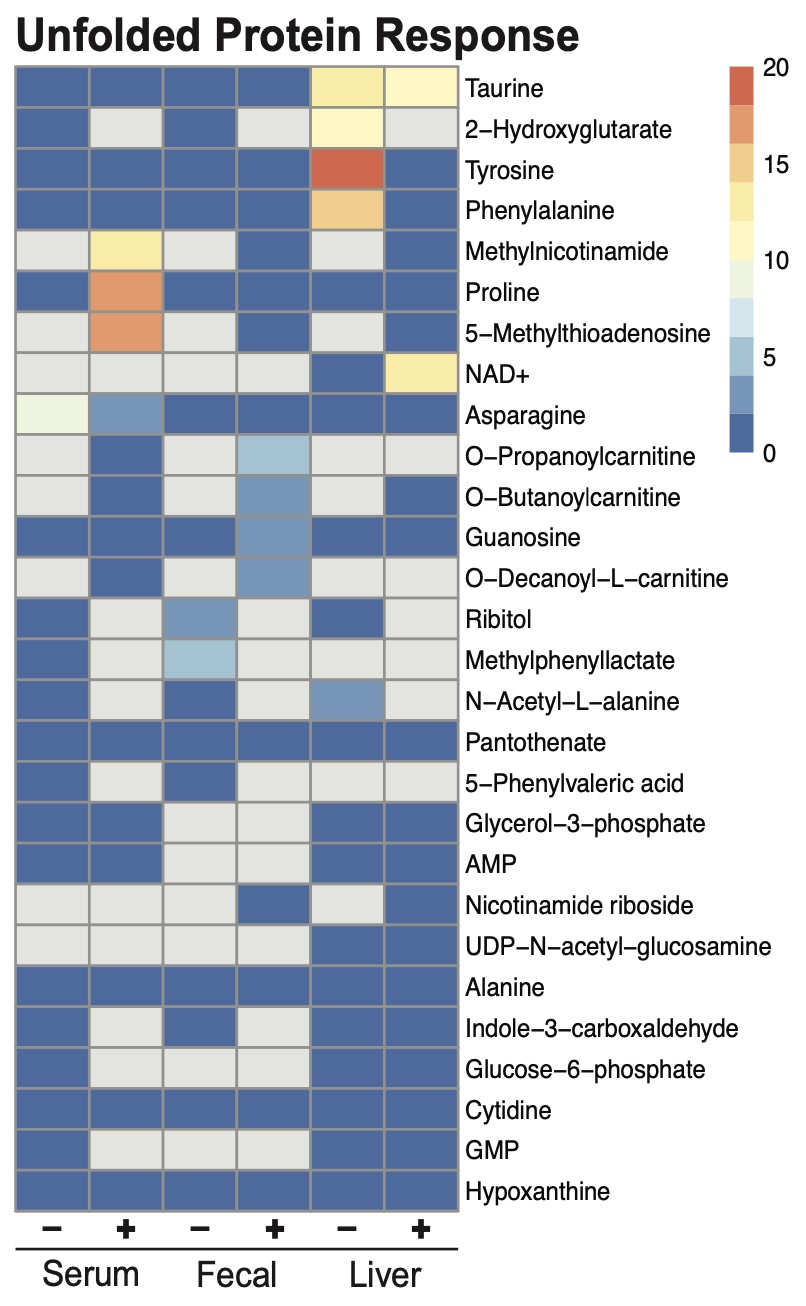
Figure: Figure 1. Effect of LGG and Trp on liver metabolome and transcriptome. A PCA of liver metabolome. B PCA of liver transcriptome.
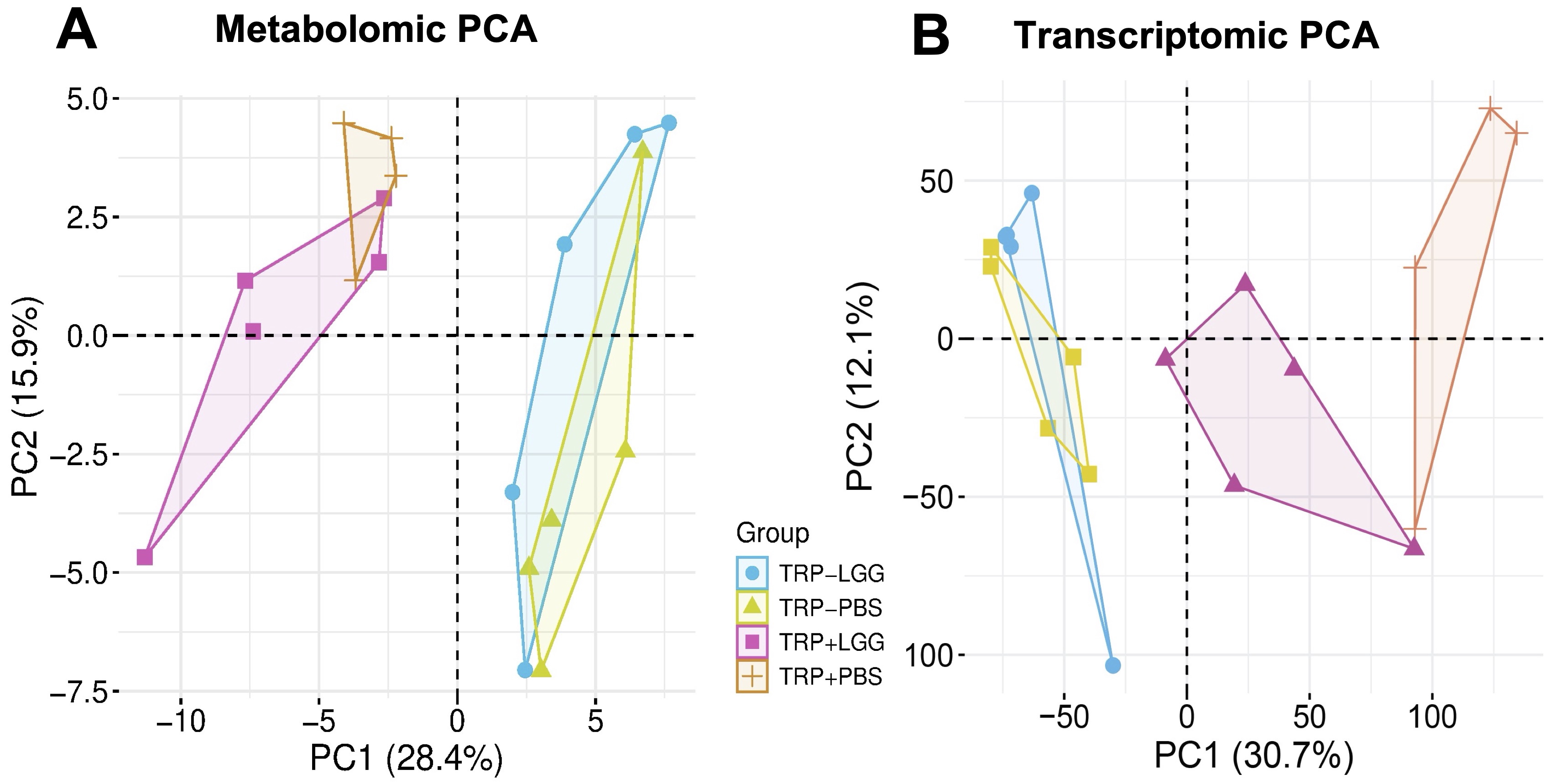
Figure: Figure 2. MeTCRA associations between metabolites and transcripts in the Unfolded Protein Response pathway in TRP⁺ mice.
Disclosures:
Amy Narakornpichit indicated no relevant financial relationships.
Jayson Antonio indicated no relevant financial relationships.
Josh Kanattu indicated no relevant financial relationships.
A.J. Justice indicated no relevant financial relationships.
Panan Suntornsaratoon indicated no relevant financial relationships.
Nan Gao indicated no relevant financial relationships.
Ronaldo Ferraris indicated no relevant financial relationships.
Amy Narakornpichit, BS1, Jayson Antonio, PhD1, Josh Kanattu, BS1, A.J. Justice, MBS1, Panan Suntornsaratoon, PhD2, Nan Gao, PhD1, Ronaldo Ferraris, PhD1. P1565 - Probiotic Lactobacillus Rhamnosus GG Modulates Dietary Tryptophan-Induced Hepatic Reprogramming in Mice, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Amy Narakornpichit, BS1, Jayson Antonio, PhD1, Josh Kanattu, BS1, A.J. Justice, MBS1, Panan Suntornsaratoon, PhD2, Nan Gao, PhD1, Ronaldo Ferraris, PhD1
1Rutgers New Jersey Medical School, Newark, NJ; 2Rutgers New Jersey Medical School, Salaya, Nakhon Pathom, Thailand
Introduction: Dietary tryptophan (Trp) shapes microbial metabolite production, and its deficiency is strongly associated with the pathogenesis of metabolic dysfunction-associated steatotic liver disease (MASLD), which affects over 100 million Americans. Probiotic bacteria have been shown to mitigate gut dysbiosis and liver inflammation in MASLD, yet how diet and probiotics interact to influence hepatic signaling remains poorly defined. We integrated transcriptomic (~18,500 genes) and metabolomic profiling to investigate how Trp availability and probiotic Lactobacillus rhamnosus GG (LGG) supplementation jointly modulate hepatic signaling networks in mice.
Methods: Germ-free mice were fed a Trp-sufficient (TRP+) or Trp-deficient (TRP-) diet for two weeks, followed by a single oral gavage of LGG or PBS (vehicle), and remained on their diets for three more weeks. Liver, serum, and fecal samples were analyzed by RNA-seq and metabolomics. Differential gene expression was assessed with DESeq2; gene set enrichment analysis identified key pathways; and metabolome-transcriptome correlation analysis (MeTCRA) revealed gene-metabolite associations.
Results: Principal component analysis revealed that Trp availability is the dominant driver of both hepatic metabolomic and transcriptomic profiles. In TRP- mice, LGG had minimal impact on gene expression, underscoring the necessity of Trp for microbiota-driven hepatic regulation. In contrast, under TRP+ conditions, LGG synergistically altered liver metabolites and substantially remodeled hepatic gene expression, activating oxidative phosphorylation, MYC/E2F-mediated proliferation, and mTOR signaling pathways. Although LGG induced only 38 differentially expressed genes in TRP+ mice (TRP+LGG vs TRP+PBS), enriched pathways were broad—spanning proliferation, barrier function, and the unfolded protein response. MeTCRA linked hepatic taurine, tyrosine, and NAD+, to these responses, highlighting the interplay between dietary amino acids, gut microbiota, and energy-regulatory circuits.
Discussion: LGG-driven hepatic effects are highly contingent on Trp sufficiency. Under adequate dietary conditions, LGG initiates a coordinated transcriptional and metabolic cascade involving immune, stress, and proliferative networks. These findings establish a critical diet–microbiome–liver axis and support the development of targeted nutritional and microbial strategies for managing MASLD and related disorders. AI-assisted language editing only; all scientific content developed by the authors.

Figure: Figure 1. Effect of LGG and Trp on liver metabolome and transcriptome. A PCA of liver metabolome. B PCA of liver transcriptome.

Figure: Figure 2. MeTCRA associations between metabolites and transcripts in the Unfolded Protein Response pathway in TRP⁺ mice.
Disclosures:
Amy Narakornpichit indicated no relevant financial relationships.
Jayson Antonio indicated no relevant financial relationships.
Josh Kanattu indicated no relevant financial relationships.
A.J. Justice indicated no relevant financial relationships.
Panan Suntornsaratoon indicated no relevant financial relationships.
Nan Gao indicated no relevant financial relationships.
Ronaldo Ferraris indicated no relevant financial relationships.
Amy Narakornpichit, BS1, Jayson Antonio, PhD1, Josh Kanattu, BS1, A.J. Justice, MBS1, Panan Suntornsaratoon, PhD2, Nan Gao, PhD1, Ronaldo Ferraris, PhD1. P1565 - Probiotic Lactobacillus Rhamnosus GG Modulates Dietary Tryptophan-Induced Hepatic Reprogramming in Mice, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

