Sunday Poster Session
Category: Liver
P1542 - A Systematic Review of Gender, Racial and Ethnic Representation in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Clinical Trials in the United States
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- SD
Shrey Dalwadi, BS
Cooper Medical School of Rowan University
Camden, NJ
Presenting Author(s)
Zehara Abidi, MS1, Avneet Singh, DO2, Shrey Dalwadi, BS3, Alexander Garcia, DO4, Sonal Kumar, MD, MPH5, Carolyn Newberry, MD6, Adam Buckholz, MD6
1Touro College of Osteopathic Medicine, Harlem, NY; 2Cooper University Health Care, Camden, NJ; 3Cooper Medical School of Rowan University, Camden, NJ; 4Cooper University Hospital, Camden, NJ; 5NewYork-Presbyterian Hospital/Weill Cornell Medical Center, New York, NY; 6NewYork-Presbyterian / Weill Cornell Medical Center, New York, NY
Introduction: Metabolic dysfunction-associated steatotic liver disease (MASLD) affects 25-30% of US adults and there are known racial and ethnic differences in disease prevalence and rates of progression. There are many ongoing trials of potential therapies, but minimal data exists to characterize participant demographics. Here we characterize the gender, ethnic and racial breakdown of US clinical trials to understand disparities in minority representation.
Methods: We conducted a systematic review of all completed trials with results on Clinicaltrials.gov from inception to March 2024. Included studies were limited to MASLD and MASLD-Cirrhosis in adults. Studies were excluded if clinical sites were outside of the US. Racial and ethnic composition of MASLD was calculated using prevalence rates and the 2020 US Census. Data pertaining to age, gender, race, and ethnicity were abstracted.
Results: 211 trials were retrieved and 51 trials with 4225 participants were included(Fig 1). The mean age was 52.14 (SD 10.66) and female (n=2288; 54.2%) participants exceeded males (n=1937; 45.8%). Ethnicity data was available in 41/51 (81%) of studies, with fewer Hispanic vs Non-Hispanic enrollees (n=3492; 37.1% vs 62.9%). Enrollment breakdown by race was reported in 50/51 studies with the largest representation among White enrollees and the lowest among Native Hawaiian/Pacific Islanders [(n= 4124; White= 3561 (86.3%), Black= 203 (4.92%), Asian= 139 (3.4%), Native American= 23 (0.6%), Native Hawaiian/Pacific Islander= 14 (0.34%), other races= 64 (1.6%)] (Fig 2). Compared to national prevalence estimates, Hispanic (37.1% vs 23.7%) and White (86.3% vs 59.7%) enrollees were over-represented, while Black (4.92% vs 9.2%) and Asian (3.4% vs 5.9%) enrollees were underrepresented.
Discussion: This review of demographics in MASLD trials suggests several key disparities. Importantly, we noted low absolute and relative Black and Asian enrollment. Diversity of trial inclusion is key to understanding biological differences in outcomes, and the limited trial data in these minorities is striking. Research identifying barriers to inclusion such as language, medical mistrust or socioeconomic factors should be studied to ensure future clinical trials recruit patients that accurately reflect the national racial and ethnic distribution of MASLD. Additionally, significant heterogeneity was noted in ethnic and racial reporting, which is critical to address especially given the increase in multi-ethnic/multi-racial backgrounds in the US.
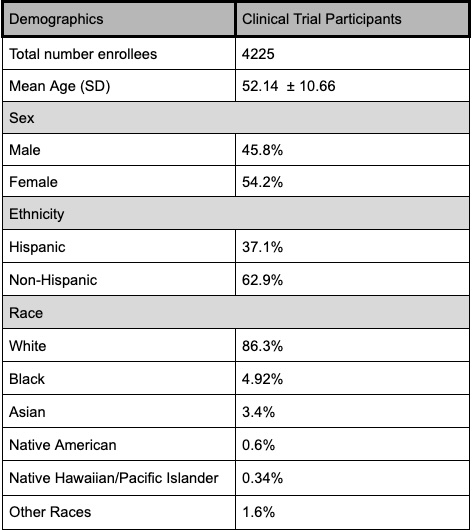
Figure: Figure 1. Demographics of Clinical Trial Participants
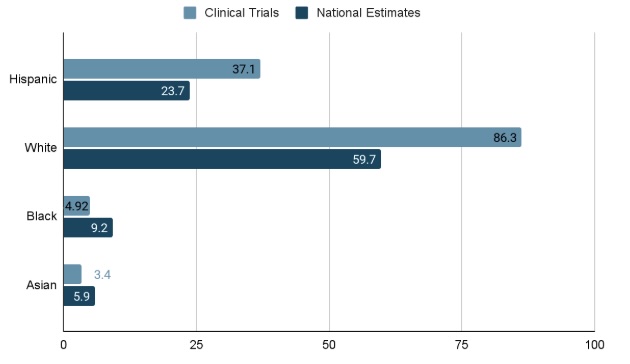
Figure: Figure 2. Comparing the Ethnic and Racial Composition of MASLD Clinical Trial Participants to National Estimates
Disclosures:
Zehara Abidi indicated no relevant financial relationships.
Avneet Singh indicated no relevant financial relationships.
Shrey Dalwadi indicated no relevant financial relationships.
Alexander Garcia indicated no relevant financial relationships.
Sonal Kumar: CymaBay Therapeutics, Inc. – Advisory Committee/Board Member. Empire Liver Foundation, LLC – Speaker. Gilead Sciences, Inc. – Speaker. Intercept Pharmaceuticals, INC – Advisory Committee/Board Member. Intercept Pharmaceuticals, INC – Speaker. Ipsen Pharma S.A.S. – Consultant. Ipsen Pharma S.A.S. – Speaker. Novo Nordisk Pharmaceuticals, Inc. – Consultant. Novo Nordisk Pharmaceuticals, Inc. – Speaker.
Carolyn Newberry: Eli Lilly & Co – Consultant.
Adam Buckholz indicated no relevant financial relationships.
Zehara Abidi, MS1, Avneet Singh, DO2, Shrey Dalwadi, BS3, Alexander Garcia, DO4, Sonal Kumar, MD, MPH5, Carolyn Newberry, MD6, Adam Buckholz, MD6. P1542 - A Systematic Review of Gender, Racial and Ethnic Representation in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Clinical Trials in the United States, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Touro College of Osteopathic Medicine, Harlem, NY; 2Cooper University Health Care, Camden, NJ; 3Cooper Medical School of Rowan University, Camden, NJ; 4Cooper University Hospital, Camden, NJ; 5NewYork-Presbyterian Hospital/Weill Cornell Medical Center, New York, NY; 6NewYork-Presbyterian / Weill Cornell Medical Center, New York, NY
Introduction: Metabolic dysfunction-associated steatotic liver disease (MASLD) affects 25-30% of US adults and there are known racial and ethnic differences in disease prevalence and rates of progression. There are many ongoing trials of potential therapies, but minimal data exists to characterize participant demographics. Here we characterize the gender, ethnic and racial breakdown of US clinical trials to understand disparities in minority representation.
Methods: We conducted a systematic review of all completed trials with results on Clinicaltrials.gov from inception to March 2024. Included studies were limited to MASLD and MASLD-Cirrhosis in adults. Studies were excluded if clinical sites were outside of the US. Racial and ethnic composition of MASLD was calculated using prevalence rates and the 2020 US Census. Data pertaining to age, gender, race, and ethnicity were abstracted.
Results: 211 trials were retrieved and 51 trials with 4225 participants were included(Fig 1). The mean age was 52.14 (SD 10.66) and female (n=2288; 54.2%) participants exceeded males (n=1937; 45.8%). Ethnicity data was available in 41/51 (81%) of studies, with fewer Hispanic vs Non-Hispanic enrollees (n=3492; 37.1% vs 62.9%). Enrollment breakdown by race was reported in 50/51 studies with the largest representation among White enrollees and the lowest among Native Hawaiian/Pacific Islanders [(n= 4124; White= 3561 (86.3%), Black= 203 (4.92%), Asian= 139 (3.4%), Native American= 23 (0.6%), Native Hawaiian/Pacific Islander= 14 (0.34%), other races= 64 (1.6%)] (Fig 2). Compared to national prevalence estimates, Hispanic (37.1% vs 23.7%) and White (86.3% vs 59.7%) enrollees were over-represented, while Black (4.92% vs 9.2%) and Asian (3.4% vs 5.9%) enrollees were underrepresented.
Discussion: This review of demographics in MASLD trials suggests several key disparities. Importantly, we noted low absolute and relative Black and Asian enrollment. Diversity of trial inclusion is key to understanding biological differences in outcomes, and the limited trial data in these minorities is striking. Research identifying barriers to inclusion such as language, medical mistrust or socioeconomic factors should be studied to ensure future clinical trials recruit patients that accurately reflect the national racial and ethnic distribution of MASLD. Additionally, significant heterogeneity was noted in ethnic and racial reporting, which is critical to address especially given the increase in multi-ethnic/multi-racial backgrounds in the US.

Figure: Figure 1. Demographics of Clinical Trial Participants

Figure: Figure 2. Comparing the Ethnic and Racial Composition of MASLD Clinical Trial Participants to National Estimates
Disclosures:
Zehara Abidi indicated no relevant financial relationships.
Avneet Singh indicated no relevant financial relationships.
Shrey Dalwadi indicated no relevant financial relationships.
Alexander Garcia indicated no relevant financial relationships.
Sonal Kumar: CymaBay Therapeutics, Inc. – Advisory Committee/Board Member. Empire Liver Foundation, LLC – Speaker. Gilead Sciences, Inc. – Speaker. Intercept Pharmaceuticals, INC – Advisory Committee/Board Member. Intercept Pharmaceuticals, INC – Speaker. Ipsen Pharma S.A.S. – Consultant. Ipsen Pharma S.A.S. – Speaker. Novo Nordisk Pharmaceuticals, Inc. – Consultant. Novo Nordisk Pharmaceuticals, Inc. – Speaker.
Carolyn Newberry: Eli Lilly & Co – Consultant.
Adam Buckholz indicated no relevant financial relationships.
Zehara Abidi, MS1, Avneet Singh, DO2, Shrey Dalwadi, BS3, Alexander Garcia, DO4, Sonal Kumar, MD, MPH5, Carolyn Newberry, MD6, Adam Buckholz, MD6. P1542 - A Systematic Review of Gender, Racial and Ethnic Representation in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Clinical Trials in the United States, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
