Sunday Poster Session
Category: Liver
P1541 - Metabolic Dysfunction-Associated Steatotic Liver Disease and Hormone Therapy for Gender-Affirming Care
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- KY
Karen Young, MD (she/her/hers)
Duke University Hospital
Durham, NC
Presenting Author(s)
Karen Young, MD1, Carly Kelley, MD, MPH1, Suresh Balu, MS, MBA1, Andy Weinhold, MPH1, Alice Parish, MSPH2, Donna Niedzwiecki, PhD2, Heather E. Parnell, MSW2, Amreen Dinani, MD, MS1
1Duke University Hospital, Durham, NC; 2Duke University Medical Center, Durham, NC
Introduction: The prevalence of transgender and gender diverse individuals has increased over the last decade. The risk of developing metabolic dysfunction-associated steatotic liver disease in this group (MASLD) is unknown. We hypothesize that testosterone therapy is associated with increased risk of MASLD, estimated by change in alanine transaminase (ALT) serum level, in transgender and gender diverse patients who were assigned female at birth.
Methods: This retrospective review involved a subgroup analysis of a Gender Medicine Registry called DREAM (Duke Research for Equitable Access to Medicine). The population of interest included all transgender and gender diverse adults who were female at birth seen in the Gender Medicine Clinic between January 1, 2018 to February 28, 2024 with at least 1 year follow up. Patients with known chronic liver diseases were excluded. The exposure variable was testosterone therapy, and the outcome of interest was ALT level evaluated at two time periods: initiation of testosterone therapy or first encounter date, and one year follow-up.
Results: 217 patients were included in the final cohort, with an average age of 26.7 years (SD 8.8) and average BMI of 30.3 kg/m2 (SD 8.5). 195 patients (89.9%) were started on testosterone on or after their first visit date. Regarding the outcome variable (Table 1), 69% of the cohort did not have baseline or 1-year follow up labs recorded (90% of the non-exposed group, and 66% of the testosterone group). The mean change in ALT over one year follow-up for group that received testosterone was 4.64 U/L (SD 13.2). Statistical analyses comparing these differences are pending at time of abstract submission.
Discussion: This study evaluates the risk of developing liver disease after institution of testosterone therapy in a traditionally underrepresented population. At baseline, most patients in this cohort did not have an elevated ALT level indicative of low probability of intrinsic liver disease or liver fibrosis. More than two-thirds of our cohort had missing data making it difficult to draw conclusions. This highlights the importance of performing regular liver enzyme tests with testosterone therapy, especially as patients age and have longer exposure to therapy. We recognize that short duration, young average age, and high percentage of missing data are major limitations of this study. Next steps for include performing individual chart reviews to look for missing laboratory data, and future studies with longer follow-up periods.
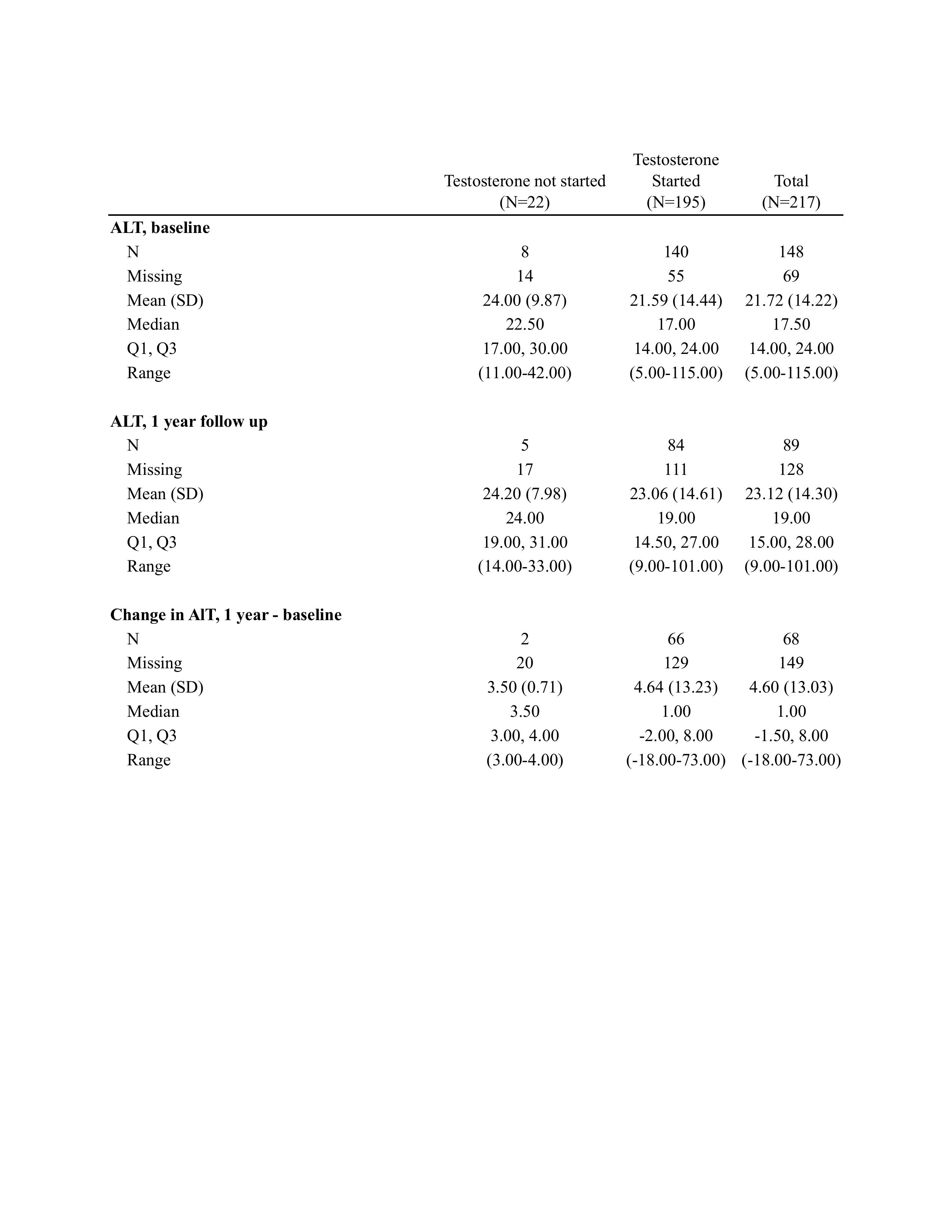
Figure: Table 1. Changes in ALT levels by testosterone therapy initiation
Disclosures:
Karen Young indicated no relevant financial relationships.
Carly Kelley indicated no relevant financial relationships.
Suresh Balu: Clinetic Inc – Stock-privately held company. CohereMed Inc – Stock-privately held company. Fullsteam Inc – Stock-privately held company.
Andy Weinhold indicated no relevant financial relationships.
Alice Parish indicated no relevant financial relationships.
Donna Niedzwiecki indicated no relevant financial relationships.
Heather Parnell indicated no relevant financial relationships.
Amreen Dinani: Madrigal Pharmaceuticals – Advisor or Review Panel Member, Consultant. NovoNordisk – Advisor or Review Panel Member, Consultant. Petauri – Advisor or Review Panel Member, Consultant.
Karen Young, MD1, Carly Kelley, MD, MPH1, Suresh Balu, MS, MBA1, Andy Weinhold, MPH1, Alice Parish, MSPH2, Donna Niedzwiecki, PhD2, Heather E. Parnell, MSW2, Amreen Dinani, MD, MS1. P1541 - Metabolic Dysfunction-Associated Steatotic Liver Disease and Hormone Therapy for Gender-Affirming Care, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Duke University Hospital, Durham, NC; 2Duke University Medical Center, Durham, NC
Introduction: The prevalence of transgender and gender diverse individuals has increased over the last decade. The risk of developing metabolic dysfunction-associated steatotic liver disease in this group (MASLD) is unknown. We hypothesize that testosterone therapy is associated with increased risk of MASLD, estimated by change in alanine transaminase (ALT) serum level, in transgender and gender diverse patients who were assigned female at birth.
Methods: This retrospective review involved a subgroup analysis of a Gender Medicine Registry called DREAM (Duke Research for Equitable Access to Medicine). The population of interest included all transgender and gender diverse adults who were female at birth seen in the Gender Medicine Clinic between January 1, 2018 to February 28, 2024 with at least 1 year follow up. Patients with known chronic liver diseases were excluded. The exposure variable was testosterone therapy, and the outcome of interest was ALT level evaluated at two time periods: initiation of testosterone therapy or first encounter date, and one year follow-up.
Results: 217 patients were included in the final cohort, with an average age of 26.7 years (SD 8.8) and average BMI of 30.3 kg/m2 (SD 8.5). 195 patients (89.9%) were started on testosterone on or after their first visit date. Regarding the outcome variable (Table 1), 69% of the cohort did not have baseline or 1-year follow up labs recorded (90% of the non-exposed group, and 66% of the testosterone group). The mean change in ALT over one year follow-up for group that received testosterone was 4.64 U/L (SD 13.2). Statistical analyses comparing these differences are pending at time of abstract submission.
Discussion: This study evaluates the risk of developing liver disease after institution of testosterone therapy in a traditionally underrepresented population. At baseline, most patients in this cohort did not have an elevated ALT level indicative of low probability of intrinsic liver disease or liver fibrosis. More than two-thirds of our cohort had missing data making it difficult to draw conclusions. This highlights the importance of performing regular liver enzyme tests with testosterone therapy, especially as patients age and have longer exposure to therapy. We recognize that short duration, young average age, and high percentage of missing data are major limitations of this study. Next steps for include performing individual chart reviews to look for missing laboratory data, and future studies with longer follow-up periods.

Figure: Table 1. Changes in ALT levels by testosterone therapy initiation
Disclosures:
Karen Young indicated no relevant financial relationships.
Carly Kelley indicated no relevant financial relationships.
Suresh Balu: Clinetic Inc – Stock-privately held company. CohereMed Inc – Stock-privately held company. Fullsteam Inc – Stock-privately held company.
Andy Weinhold indicated no relevant financial relationships.
Alice Parish indicated no relevant financial relationships.
Donna Niedzwiecki indicated no relevant financial relationships.
Heather Parnell indicated no relevant financial relationships.
Amreen Dinani: Madrigal Pharmaceuticals – Advisor or Review Panel Member, Consultant. NovoNordisk – Advisor or Review Panel Member, Consultant. Petauri – Advisor or Review Panel Member, Consultant.
Karen Young, MD1, Carly Kelley, MD, MPH1, Suresh Balu, MS, MBA1, Andy Weinhold, MPH1, Alice Parish, MSPH2, Donna Niedzwiecki, PhD2, Heather E. Parnell, MSW2, Amreen Dinani, MD, MS1. P1541 - Metabolic Dysfunction-Associated Steatotic Liver Disease and Hormone Therapy for Gender-Affirming Care, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
