Sunday Poster Session
Category: Liver
P1492 - Effectiveness of Terlipressin in Hepatorenal Syndrome-Acute Kidney Injury Reversal: A Systematic Review and Meta-Analysis of Randomized Trials
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- MW
Mahader N. Wossene, MD (he/him/his)
MacNeal Hospital Loyola Medicine
Berwyn, IL
Presenting Author(s)
Mahader N. Wossene, MD1, Vaishnavi K. Modi, MD2, Elsa W. Mamo, MD3, Selome T. Berhanu, MD3, Genet Hagos. Weldemichael, MD1, Anum Nawaz, MBBS, MD2, Zohaib Haque, DO4
1MacNeal Hospital Loyola Medicine, Berwyn, IL; 2MacNeal Hospital Loyola Medicine, Oak Park, IL; 3addis ababa university, Addis Ababa, Adis Abeba, Ethiopia; 4MacNeal Hospital Loyola Medicine, Chicago, IL
Introduction: Hepatorenal syndrome (HRS) is a type of functional renal impairment that specifically occurs in patients with advanced liver disease. HRS carries an extremely poor prognosis and high mortality despite its potential reversibility with pharmacologic treatment and liver transplant. Terlipressin, a vasopressin analog, has recently gained FDA approval for HRS, but its overall efficacy remains debated.
Methods: We performed a systematic review and meta-analysis of randomized controlled trials (RCTs) published between 2005 and 2025. Databases searched included Medline, Embase, Cochrane Library, and Scopus. Studies included adult patients with Hepatorenal syndrome-acute kidney injury (HRS-AKI) or type 1 HRS who received terlipressin or a comparator (placebo or other vasoconstrictors). Trials without a clear definition of HRS reversal were not included. The primary outcome was HRS reversal. Secondary outcome included mortality. Random-effects meta-analysis to estimate risk ratio (RR) for HRS reversal was performed using the REML method with continuity correction for zero-event studies. Additionally, mortality was analyzed. Publication bias was assessed using funnel plots and the trim-and-fill method. Cochrane risk of bias assessment tool was used to assess for quality.
Results: Seven RCTs involving a total of 763 subjects with a diagnosis of type 1 HRS were included. HRS reversal defined as a value of creatinine less than 1.5 in those with a diagnosis of HRS-AKI or type 1 HRS. The final pooled analysis showed a higher rate of HRS reversal (RR of 1.53, 95% confidence interval (CI) [1.07 – 2.19]; P = 0.02) with terlipressin than competitor. The result was statistically significant. Trim-and-fill analysis suggestive for publication bias. Mortality does not show significant improvement (RR of 0.999, 95% confidence interval (CI) [0.996 – 1.001]; P = 1.53) with the terlipressin as compared to competitor.
Discussion: Terlipressin significantly improves the likelihood of HRS reversal compared to placebo. However, trim-and-fill analysis suggests potential publication bias, which may overestimate the benefit. Terlipressin is not seen to have significant mortality benefits. Further studies are warranted to examine long-term survival and safety.
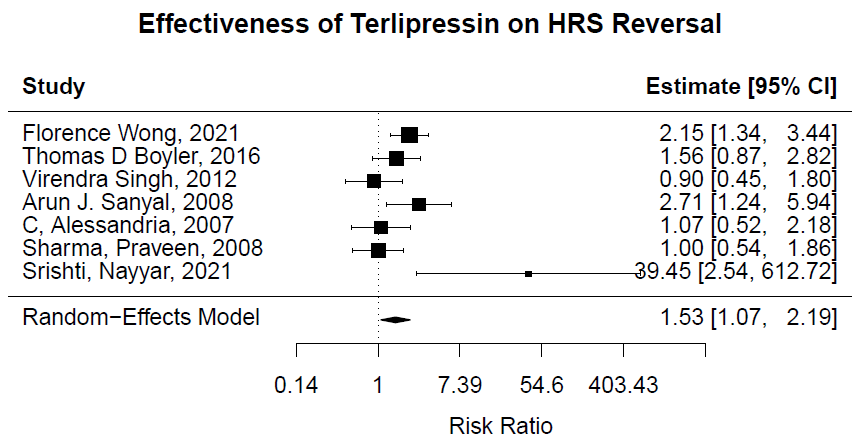
Figure: Forrest plot indicating individual and overall effect size of the seven included studies.
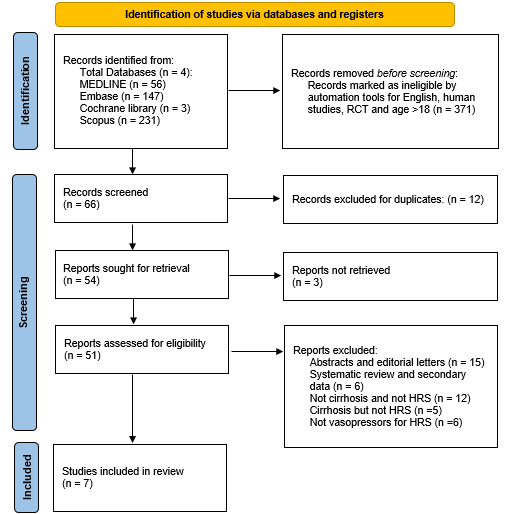
Figure: PRISMA flow diagram
Disclosures:
Mahader Wossene indicated no relevant financial relationships.
Vaishnavi Modi indicated no relevant financial relationships.
Elsa Mamo indicated no relevant financial relationships.
Selome Berhanu indicated no relevant financial relationships.
Genet Weldemichael indicated no relevant financial relationships.
Anum Nawaz indicated no relevant financial relationships.
Zohaib Haque indicated no relevant financial relationships.
Mahader N. Wossene, MD1, Vaishnavi K. Modi, MD2, Elsa W. Mamo, MD3, Selome T. Berhanu, MD3, Genet Hagos. Weldemichael, MD1, Anum Nawaz, MBBS, MD2, Zohaib Haque, DO4. P1492 - Effectiveness of Terlipressin in Hepatorenal Syndrome-Acute Kidney Injury Reversal: A Systematic Review and Meta-Analysis of Randomized Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1MacNeal Hospital Loyola Medicine, Berwyn, IL; 2MacNeal Hospital Loyola Medicine, Oak Park, IL; 3addis ababa university, Addis Ababa, Adis Abeba, Ethiopia; 4MacNeal Hospital Loyola Medicine, Chicago, IL
Introduction: Hepatorenal syndrome (HRS) is a type of functional renal impairment that specifically occurs in patients with advanced liver disease. HRS carries an extremely poor prognosis and high mortality despite its potential reversibility with pharmacologic treatment and liver transplant. Terlipressin, a vasopressin analog, has recently gained FDA approval for HRS, but its overall efficacy remains debated.
Methods: We performed a systematic review and meta-analysis of randomized controlled trials (RCTs) published between 2005 and 2025. Databases searched included Medline, Embase, Cochrane Library, and Scopus. Studies included adult patients with Hepatorenal syndrome-acute kidney injury (HRS-AKI) or type 1 HRS who received terlipressin or a comparator (placebo or other vasoconstrictors). Trials without a clear definition of HRS reversal were not included. The primary outcome was HRS reversal. Secondary outcome included mortality. Random-effects meta-analysis to estimate risk ratio (RR) for HRS reversal was performed using the REML method with continuity correction for zero-event studies. Additionally, mortality was analyzed. Publication bias was assessed using funnel plots and the trim-and-fill method. Cochrane risk of bias assessment tool was used to assess for quality.
Results: Seven RCTs involving a total of 763 subjects with a diagnosis of type 1 HRS were included. HRS reversal defined as a value of creatinine less than 1.5 in those with a diagnosis of HRS-AKI or type 1 HRS. The final pooled analysis showed a higher rate of HRS reversal (RR of 1.53, 95% confidence interval (CI) [1.07 – 2.19]; P = 0.02) with terlipressin than competitor. The result was statistically significant. Trim-and-fill analysis suggestive for publication bias. Mortality does not show significant improvement (RR of 0.999, 95% confidence interval (CI) [0.996 – 1.001]; P = 1.53) with the terlipressin as compared to competitor.
Discussion: Terlipressin significantly improves the likelihood of HRS reversal compared to placebo. However, trim-and-fill analysis suggests potential publication bias, which may overestimate the benefit. Terlipressin is not seen to have significant mortality benefits. Further studies are warranted to examine long-term survival and safety.

Figure: Forrest plot indicating individual and overall effect size of the seven included studies.

Figure: PRISMA flow diagram
Disclosures:
Mahader Wossene indicated no relevant financial relationships.
Vaishnavi Modi indicated no relevant financial relationships.
Elsa Mamo indicated no relevant financial relationships.
Selome Berhanu indicated no relevant financial relationships.
Genet Weldemichael indicated no relevant financial relationships.
Anum Nawaz indicated no relevant financial relationships.
Zohaib Haque indicated no relevant financial relationships.
Mahader N. Wossene, MD1, Vaishnavi K. Modi, MD2, Elsa W. Mamo, MD3, Selome T. Berhanu, MD3, Genet Hagos. Weldemichael, MD1, Anum Nawaz, MBBS, MD2, Zohaib Haque, DO4. P1492 - Effectiveness of Terlipressin in Hepatorenal Syndrome-Acute Kidney Injury Reversal: A Systematic Review and Meta-Analysis of Randomized Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
