Sunday Poster Session
Category: Interventional Endoscopy
P1439 - Pilot Study of a Novel Device for Endoscopic Vacuum Therapy for Anastomotic Leak
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- MC
Maria A. Cassera, MD
Digestive Health Center University of Washington Medical Center
Seattle, WA
Presenting Author(s)
Award: ACG Case Reports Journal Award (Trainee)
Award: ACG Presidential Poster Award
Maria A. Cassera, MD, Liam Hilson, MD, Anand C. Baxi, MD, Anand Singla, MD, Michael Saunders, MD, Adam W. Templeton, MD
Digestive Health Center University of Washington Medical Center, Seattle, WA
Introduction: Gastrointestinal leaks are the result of perforation along the interior wall of the GI tract due to disease, trauma, or surgical complications. GI leaks increase the risk of mortality, infection, and hospital readmission. To date, stents have been the primary mode of treatment for a GI leak, however, wound vacuum-assisted closure (VAC) systems have been introduced as a method for sealing both external and internal wounds and leaks. Endoscopic vacuum therapy (EVT) is an emerging technology utilizing wound VAC systems for GI leaks. We have developed a novel EVT device consisting of a compressed pellet sponge and tube kit, which features a sponge encased in a dissolvable shell which expands upon positioning at the leak site (Figure 1). We present a single-center prospective pilot study to evaluate the safety and efficacy of this novel EVT device in treatment of GI leaks.
Case Description/
Methods: Patients diagnosed with an esophagogastric anastomotic leak were identified, and enrolled in a prospective trial for a novel EVT device. The EVT device is guided through the esophagus and placed at the defect site under direct endoscopic visualization, and the external end is connected to a wound VAC unit which provides a negative pressure environment. The EVT device is exchanged every 3-7 days per protocol until the anastomotic leak has healed (Figure 2). Patient demographics, intraprocedural data, and outcomes data were collected and analyzed. Safety was defined as avoiding severe adverse events related to the EVT device during use. Efficacy was defined as the achievement of complete healing of the anastomotic leak using contrast-enhanced CT.
A 65 year-old man with a history of gastroesophageal junction carcinoma who underwent laparoscopic trans-hiatal esophagogastrostomy with a course complicated by anastomotic leak was enrolled in the pilot study. EVT was initiated on post-operative day (POD) #6, and the EVT device was exchanged on POD #12, POD #16, and POD #20. Mean procedure time was 25.6-min (range: 24-30min). Contrast-enhanced CT imaging demonstrated complete resolution of the anastomotic leak on POD #26, and the patient was discharged on POD #28. There were no adverse events related to the novel EVT device during the trial, or at 1 month follow up.
Discussion: This case demonstrates that this novel EVT device provides a feasible and safe therapy for healing anastomotic leak.
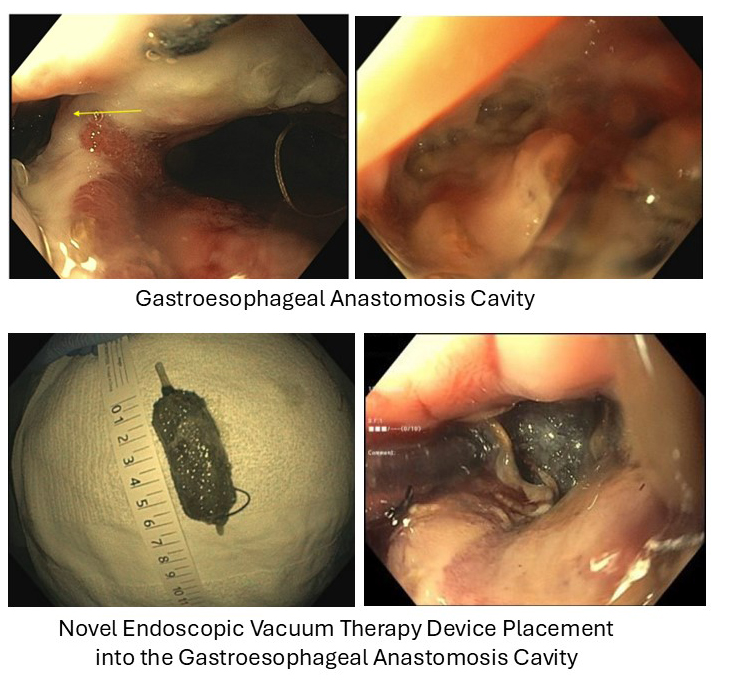
Figure: Novel Endoscopic Vacuum Therapy Device
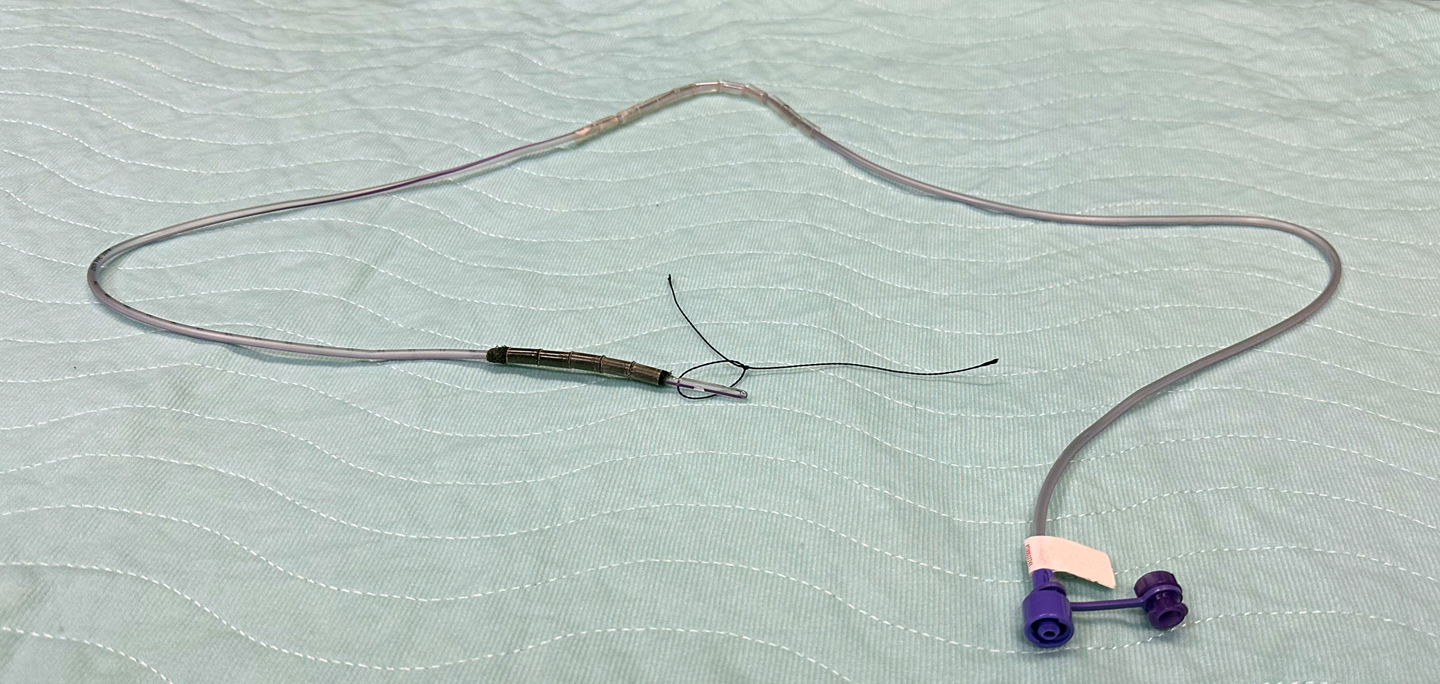
Figure: The gastroesophageal anastomosis cavity, and placement of the novel endoscopic vacuum therapy device.
Disclosures:
Maria Cassera indicated no relevant financial relationships.
Liam Hilson indicated no relevant financial relationships.
Anand Baxi indicated no relevant financial relationships.
Anand Singla indicated no relevant financial relationships.
Michael Saunders indicated no relevant financial relationships.
Adam Templeton indicated no relevant financial relationships.
Maria A. Cassera, MD, Liam Hilson, MD, Anand C. Baxi, MD, Anand Singla, MD, Michael Saunders, MD, Adam W. Templeton, MD. P1439 - Pilot Study of a Novel Device for Endoscopic Vacuum Therapy for Anastomotic Leak, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Award: ACG Presidential Poster Award
Maria A. Cassera, MD, Liam Hilson, MD, Anand C. Baxi, MD, Anand Singla, MD, Michael Saunders, MD, Adam W. Templeton, MD
Digestive Health Center University of Washington Medical Center, Seattle, WA
Introduction: Gastrointestinal leaks are the result of perforation along the interior wall of the GI tract due to disease, trauma, or surgical complications. GI leaks increase the risk of mortality, infection, and hospital readmission. To date, stents have been the primary mode of treatment for a GI leak, however, wound vacuum-assisted closure (VAC) systems have been introduced as a method for sealing both external and internal wounds and leaks. Endoscopic vacuum therapy (EVT) is an emerging technology utilizing wound VAC systems for GI leaks. We have developed a novel EVT device consisting of a compressed pellet sponge and tube kit, which features a sponge encased in a dissolvable shell which expands upon positioning at the leak site (Figure 1). We present a single-center prospective pilot study to evaluate the safety and efficacy of this novel EVT device in treatment of GI leaks.
Case Description/
Methods: Patients diagnosed with an esophagogastric anastomotic leak were identified, and enrolled in a prospective trial for a novel EVT device. The EVT device is guided through the esophagus and placed at the defect site under direct endoscopic visualization, and the external end is connected to a wound VAC unit which provides a negative pressure environment. The EVT device is exchanged every 3-7 days per protocol until the anastomotic leak has healed (Figure 2). Patient demographics, intraprocedural data, and outcomes data were collected and analyzed. Safety was defined as avoiding severe adverse events related to the EVT device during use. Efficacy was defined as the achievement of complete healing of the anastomotic leak using contrast-enhanced CT.
A 65 year-old man with a history of gastroesophageal junction carcinoma who underwent laparoscopic trans-hiatal esophagogastrostomy with a course complicated by anastomotic leak was enrolled in the pilot study. EVT was initiated on post-operative day (POD) #6, and the EVT device was exchanged on POD #12, POD #16, and POD #20. Mean procedure time was 25.6-min (range: 24-30min). Contrast-enhanced CT imaging demonstrated complete resolution of the anastomotic leak on POD #26, and the patient was discharged on POD #28. There were no adverse events related to the novel EVT device during the trial, or at 1 month follow up.
Discussion: This case demonstrates that this novel EVT device provides a feasible and safe therapy for healing anastomotic leak.

Figure: Novel Endoscopic Vacuum Therapy Device

Figure: The gastroesophageal anastomosis cavity, and placement of the novel endoscopic vacuum therapy device.
Disclosures:
Maria Cassera indicated no relevant financial relationships.
Liam Hilson indicated no relevant financial relationships.
Anand Baxi indicated no relevant financial relationships.
Anand Singla indicated no relevant financial relationships.
Michael Saunders indicated no relevant financial relationships.
Adam Templeton indicated no relevant financial relationships.
Maria A. Cassera, MD, Liam Hilson, MD, Anand C. Baxi, MD, Anand Singla, MD, Michael Saunders, MD, Adam W. Templeton, MD. P1439 - Pilot Study of a Novel Device for Endoscopic Vacuum Therapy for Anastomotic Leak, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

