Sunday Poster Session
Category: Interventional Endoscopy
P1412 - Analysis of Reported Gastrostomy Tube Problems and Patient Related Adverse Events Using the FDA MAUDE Database
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Hussam Almasri, MD, MRCP(UK)
University of North Dakota, School of Medicine and Health Sciences
Fargo, ND
Presenting Author(s)
Hussam Almasri, MD, MRCP(UK)1, Rahul Karna, MD2, Matthew Krafft, MD3, Stuart K. Amateau, MD, PhD2
1University of North Dakota, School of Medicine and Health Sciences, Fargo, ND; 2University of Minnesota Medical Center, Minneapolis, MN; 3West Virginia University School of Medicine, Morgantown, WV
Introduction: Gastrostomy tubes (G-tube) are used for enteral nutrition in patients with swallowing disorders, neurological conditions, or other diseases requiring long-term nutritional support. They can be placed by endoscopy (percutaneous endoscopic gastrostomy or PEG) with pull or push methods, or using interventional radiology techniques. By utilizing the Manufacturer and User Facility Device Experience (MAUDE) database, this study comprehensively analyzes the range and frequency of G-tube adverse events (AEs) reported over the past decade.
Methods: A retrospective review of the MAUDE database identified all G-tube AEs up to January 2025. Each report included a unique number, event date, event type, event narrative, manufacturer, device’s brand, device’s problems, and patient-related adverse outcomes. As available, additional data about device type, such as button, device size, and insertion method, were collected. Device problems were grouped into categories. Descriptive statistics and trend analyses were performed on the collected data.
Results: 1761 total reports were included in the analyses. Peak frequency was noted in 2015 and 2016, with no statistically significant trend observed in frequency by linear regression. Recorded event types included 1255 malfunctions, 478 injuries, and 28 deaths. The latter was mostly secondary to peritonitis and/or perforation. The most frequently reported device problem was balloon damage in 302 cases, followed by detachment of the device or a device component in 293 cases. "Break" was noted in 226 reports. Most reports noted no impact on the patient; that was in 1178/1761 (66.89%) of reports. Insertion site infection was the most frequently reported patient-related AE in 6.98% (123/1761), followed by "foreign body in patient" in 6.81% (120/1761). Endovive Safety PEG Kit by Boston Scientific was the most frequently reported (361), with the most common problem being detachment. Balloon problems and fluid leaks were more common in button devices, while material disruption was more common in non-button devices (P< 0.001).
Discussion: Our analyses showed that detachment of a tube component and balloon malfunction were the most common modes of failure. Manufacturers should consider altering tube components, possibly by increasing material integrity. Track-related infection was the most common patient-related AEs, highlighting the importance of periprocedural antibiotics and periodical clinical assessments.
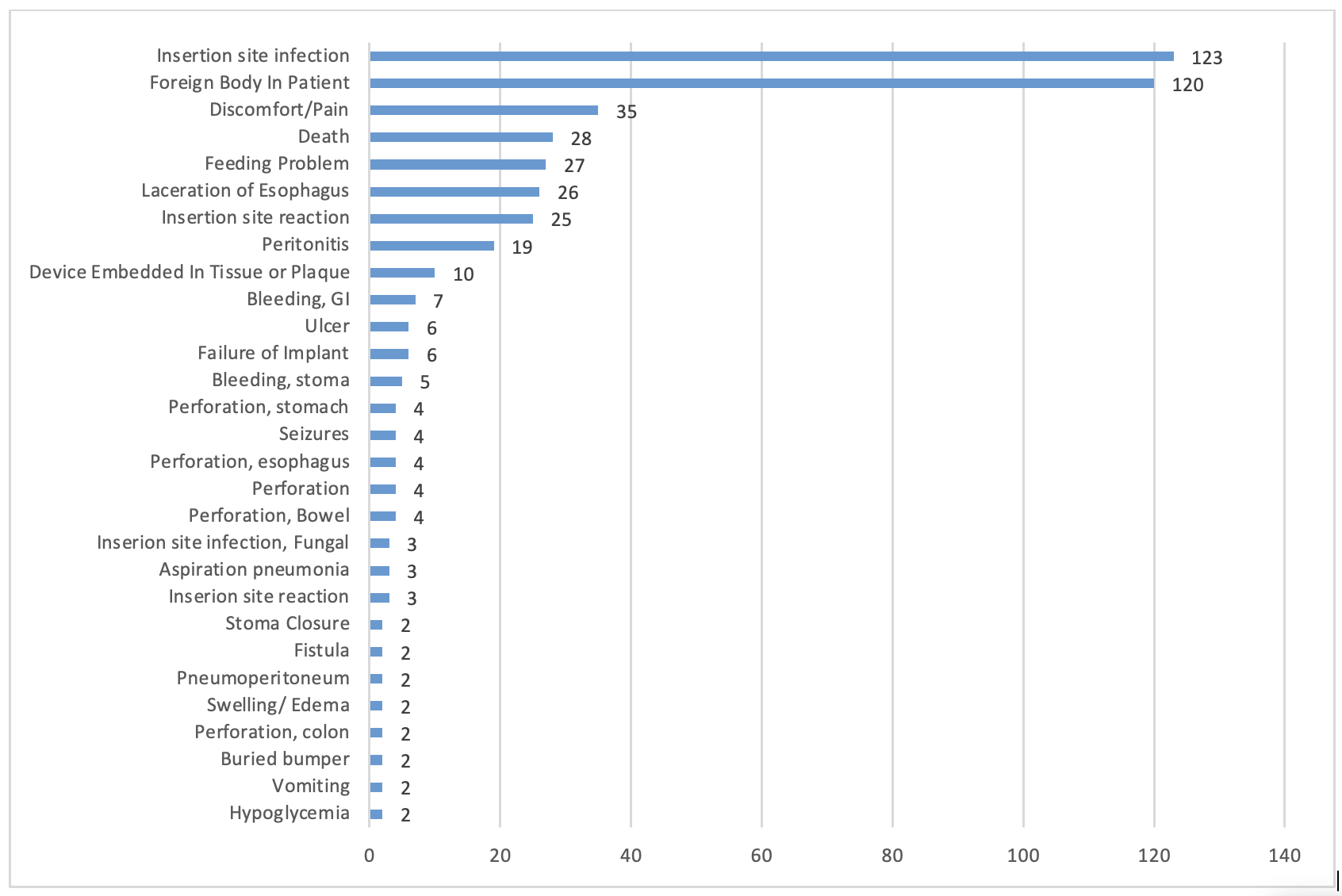
Figure: PEG/G-Tube related AEs and event type trend with time
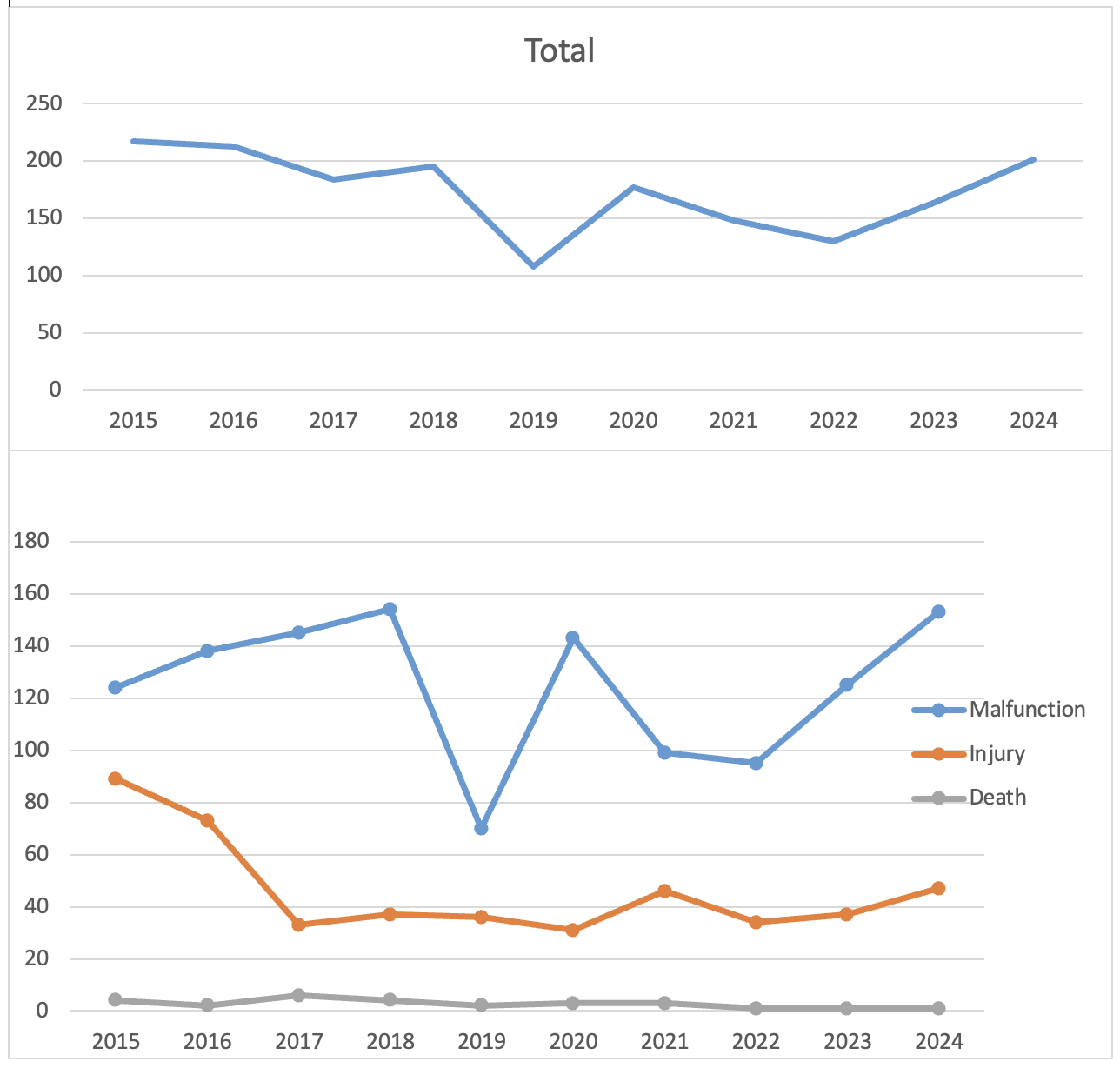
Figure: Patient-related AEs by frequency
Disclosures:
Hussam Almasri indicated no relevant financial relationships.
Rahul Karna indicated no relevant financial relationships.
Matthew Krafft indicated no relevant financial relationships.
Stuart Amateau: BSC – Consultant. Cook – Consultant. Endo-Therapeutics – Consultant. Merit – Consultant. Olympus – Advisor or Review Panel Member, Consultant. Provation – Advisory Committee/Board Member. Steris – Consultant.
Hussam Almasri, MD, MRCP(UK)1, Rahul Karna, MD2, Matthew Krafft, MD3, Stuart K. Amateau, MD, PhD2. P1412 - Analysis of Reported Gastrostomy Tube Problems and Patient Related Adverse Events Using the FDA MAUDE Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of North Dakota, School of Medicine and Health Sciences, Fargo, ND; 2University of Minnesota Medical Center, Minneapolis, MN; 3West Virginia University School of Medicine, Morgantown, WV
Introduction: Gastrostomy tubes (G-tube) are used for enteral nutrition in patients with swallowing disorders, neurological conditions, or other diseases requiring long-term nutritional support. They can be placed by endoscopy (percutaneous endoscopic gastrostomy or PEG) with pull or push methods, or using interventional radiology techniques. By utilizing the Manufacturer and User Facility Device Experience (MAUDE) database, this study comprehensively analyzes the range and frequency of G-tube adverse events (AEs) reported over the past decade.
Methods: A retrospective review of the MAUDE database identified all G-tube AEs up to January 2025. Each report included a unique number, event date, event type, event narrative, manufacturer, device’s brand, device’s problems, and patient-related adverse outcomes. As available, additional data about device type, such as button, device size, and insertion method, were collected. Device problems were grouped into categories. Descriptive statistics and trend analyses were performed on the collected data.
Results: 1761 total reports were included in the analyses. Peak frequency was noted in 2015 and 2016, with no statistically significant trend observed in frequency by linear regression. Recorded event types included 1255 malfunctions, 478 injuries, and 28 deaths. The latter was mostly secondary to peritonitis and/or perforation. The most frequently reported device problem was balloon damage in 302 cases, followed by detachment of the device or a device component in 293 cases. "Break" was noted in 226 reports. Most reports noted no impact on the patient; that was in 1178/1761 (66.89%) of reports. Insertion site infection was the most frequently reported patient-related AE in 6.98% (123/1761), followed by "foreign body in patient" in 6.81% (120/1761). Endovive Safety PEG Kit by Boston Scientific was the most frequently reported (361), with the most common problem being detachment. Balloon problems and fluid leaks were more common in button devices, while material disruption was more common in non-button devices (P< 0.001).
Discussion: Our analyses showed that detachment of a tube component and balloon malfunction were the most common modes of failure. Manufacturers should consider altering tube components, possibly by increasing material integrity. Track-related infection was the most common patient-related AEs, highlighting the importance of periprocedural antibiotics and periodical clinical assessments.

Figure: PEG/G-Tube related AEs and event type trend with time

Figure: Patient-related AEs by frequency
Disclosures:
Hussam Almasri indicated no relevant financial relationships.
Rahul Karna indicated no relevant financial relationships.
Matthew Krafft indicated no relevant financial relationships.
Stuart Amateau: BSC – Consultant. Cook – Consultant. Endo-Therapeutics – Consultant. Merit – Consultant. Olympus – Advisor or Review Panel Member, Consultant. Provation – Advisory Committee/Board Member. Steris – Consultant.
Hussam Almasri, MD, MRCP(UK)1, Rahul Karna, MD2, Matthew Krafft, MD3, Stuart K. Amateau, MD, PhD2. P1412 - Analysis of Reported Gastrostomy Tube Problems and Patient Related Adverse Events Using the FDA MAUDE Database, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
