Sunday Poster Session
Category: Interventional Endoscopy
P1366 - Safety of ERCP in Cancer Patients Taking Long-Acting Anti-VEGF Agents
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- GW
George Wahba, MD
The University of Texas MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
George Wahba, MD, Filippos Koutroumpakis, MD, Emmanuel Coronel, MD, Phillip S.. Ge, MD, William Ross, MD, Brian Weston, MD, Jeffrey H.. Lee, MD, MPH
The University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Anti-VEGF agents are effective in the management of colorectal cancer and other gastrointestinal malignancies but are associated with gastrointestinal bleeding and perforation. Weeks of pre- and post-surgical treatment interruption are suggested to avoid post-operative wound healing complications. There is minimal guidance and scarce data on the safety of these agents before and after ERCP. The aim of this study was to review ERCP outcomes in patients taking these agents.
Methods: A retrospective review was performed of ERCP outcomes over a seven-year period (2016-2023) in patients taking long-acting anti-VEGF agents within 8 weeks of ERCP at our tertiary cancer center.
Results: Seventy-eight patients undergoing 148 ERCPs were included. Mean age was 55.2 years and 62.8% were male. Most patients were on bevacizumab (82.1%). Sphincterotomy was performed in 31.8% and biliary dilation in 37.2%. The technical and clinical success rates of ERCP were 96.6% (95% CI: 92.2-98.9%) and 69.3% (95% CI: 60.9-76.9%) respectively. The cumulative risk of adverse events post-ERCP was 29.1% (95% CI: 21.9-37.1%), the most common being cholangitis (8.1% [95% CI: 4.3-13.7%]) and bleeding (5.4% [95% CI: 2.4-10.3%]). Perforation occurred in one patient (0.7% [95% CI: 0.02-3.7%]) who was managed conservatively. Median survival post-ERCP was 108 days (95% CI: 71-169 days). On logistic regression, recent dosing (receiving an anti-VEGF dose within 2 weeks of ERCP) and intensive dosing (receiving more than 2 doses within 8 weeks of ERCP) were not independent predictors of an adverse event occurring post-ERCP (Figure 1).
Discussion: Performing ERCP in patients on anti-VEGF agents may be associated with relatively increased risks of cholangitis and bleeding. Recent and more intensive anti-VEGF dosing do not seem to independently increase the risk of adverse events.
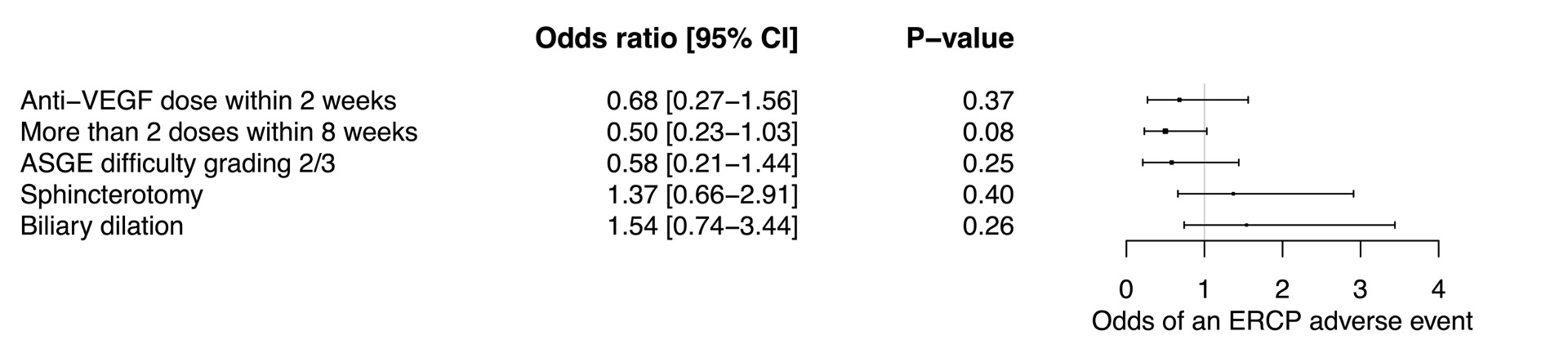
Figure: Figure 1. Multivariate analysis of odds of an adverse event occurring after ERCP.
Disclosures:
George Wahba indicated no relevant financial relationships.
Filippos Koutroumpakis indicated no relevant financial relationships.
Emmanuel Coronel indicated no relevant financial relationships.
Phillip Ge: Aspero Medical – Consultant. Boston Scientific – Consultant. Fujifilm Medical Systems – Consultant. Neptune Medical – Consultant. Ovesco Endoscopy USA – Consultant. UpToDate – Royalties.
William Ross indicated no relevant financial relationships.
Brian Weston indicated no relevant financial relationships.
Jeffrey Lee indicated no relevant financial relationships.
George Wahba, MD, Filippos Koutroumpakis, MD, Emmanuel Coronel, MD, Phillip S.. Ge, MD, William Ross, MD, Brian Weston, MD, Jeffrey H.. Lee, MD, MPH. P1366 - Safety of ERCP in Cancer Patients Taking Long-Acting Anti-VEGF Agents, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
The University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Anti-VEGF agents are effective in the management of colorectal cancer and other gastrointestinal malignancies but are associated with gastrointestinal bleeding and perforation. Weeks of pre- and post-surgical treatment interruption are suggested to avoid post-operative wound healing complications. There is minimal guidance and scarce data on the safety of these agents before and after ERCP. The aim of this study was to review ERCP outcomes in patients taking these agents.
Methods: A retrospective review was performed of ERCP outcomes over a seven-year period (2016-2023) in patients taking long-acting anti-VEGF agents within 8 weeks of ERCP at our tertiary cancer center.
Results: Seventy-eight patients undergoing 148 ERCPs were included. Mean age was 55.2 years and 62.8% were male. Most patients were on bevacizumab (82.1%). Sphincterotomy was performed in 31.8% and biliary dilation in 37.2%. The technical and clinical success rates of ERCP were 96.6% (95% CI: 92.2-98.9%) and 69.3% (95% CI: 60.9-76.9%) respectively. The cumulative risk of adverse events post-ERCP was 29.1% (95% CI: 21.9-37.1%), the most common being cholangitis (8.1% [95% CI: 4.3-13.7%]) and bleeding (5.4% [95% CI: 2.4-10.3%]). Perforation occurred in one patient (0.7% [95% CI: 0.02-3.7%]) who was managed conservatively. Median survival post-ERCP was 108 days (95% CI: 71-169 days). On logistic regression, recent dosing (receiving an anti-VEGF dose within 2 weeks of ERCP) and intensive dosing (receiving more than 2 doses within 8 weeks of ERCP) were not independent predictors of an adverse event occurring post-ERCP (Figure 1).
Discussion: Performing ERCP in patients on anti-VEGF agents may be associated with relatively increased risks of cholangitis and bleeding. Recent and more intensive anti-VEGF dosing do not seem to independently increase the risk of adverse events.

Figure: Figure 1. Multivariate analysis of odds of an adverse event occurring after ERCP.
Disclosures:
George Wahba indicated no relevant financial relationships.
Filippos Koutroumpakis indicated no relevant financial relationships.
Emmanuel Coronel indicated no relevant financial relationships.
Phillip Ge: Aspero Medical – Consultant. Boston Scientific – Consultant. Fujifilm Medical Systems – Consultant. Neptune Medical – Consultant. Ovesco Endoscopy USA – Consultant. UpToDate – Royalties.
William Ross indicated no relevant financial relationships.
Brian Weston indicated no relevant financial relationships.
Jeffrey Lee indicated no relevant financial relationships.
George Wahba, MD, Filippos Koutroumpakis, MD, Emmanuel Coronel, MD, Phillip S.. Ge, MD, William Ross, MD, Brian Weston, MD, Jeffrey H.. Lee, MD, MPH. P1366 - Safety of ERCP in Cancer Patients Taking Long-Acting Anti-VEGF Agents, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
