Sunday Poster Session
Category: Infections and Microbiome
P1291 - Combination Therapy Improves Outcomes in Chronic Hepatitis B: A Systematic Review
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- PB
Pinky Bai, MD (she/her/hers)
Howard University Hospital
Washington, DC
Presenting Author(s)
Pinky Bai, MD1, Ali Chand, MD1, Aasta Kumari, MD2, Mohammad Hassan, MD1, Musa Khalil, MD1, Nakul Ganju, MD3, Sunny Kumar, MD4, Bisma Farooq, MBBS5, Angesom Kibreab, MD1
1Howard University Hospital, Washington, DC; 2North Central Bronx Hospital, New York, NY; 3Department of Medicine, Howard University Hospital, Washington, DC; 4Wright Center for Graduate Medical Education, Scranton, PA; 5Shaikh Khalifa Bin Zayed Al Nahyan Medical and Dental Collage, Washington, DC
Introduction: Chronic Hepatitis B virus (CHB virus) affects over 250 million individuals globally and remains a leading cause of liver-related morbidity and mortality, including cirrhosis and hepatocellular carcinoma (HCC). The primary goal of antiviral treatment is sustained viral suppression to prevent disease progression. This systematic review compares the efficacy and safety of monotherapy versus combination therapy in CHB, focusing on virological and serological outcomes.
Methods: A systematic literature search was conducted using PubMed, EMBASE, and Cochrane databases for peer-reviewed studies published from 2013 to 2023. Eligible studies included randomized controlled trials (RCTs), cohort studies, and meta-analyses evaluating nucleoside/nucleotide analogues or biologics, either as monotherapy or in combination, in adult patients with CHB. Study selection followed the PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Of 965 records screened, 33 studies met inclusion criteria. Data were extracted on HBsAg clearance, HBV DNA suppression, ALT normalization, and adverse events.
Results: Combination therapy—most commonly involving pegylated interferon (PEG-IFN) with tenofovir or entecavir—demonstrated superior efficacy compared to monotherapy. HBsAg seroclearance was observed in 12–28% of patients receiving combination therapy, compared to 2–10% in monotherapy groups. HBV DNA suppression to undetectable levels was achieved in 78–95% of patients on combination therapy, versus 60–80% on monotherapy. Combination regimens also correlated with a lower incidence of HCC over long-term follow-up. Adverse event rates were similar across both treatment groups in over 90% of studies.
Discussion: The synergistic effects of combination therapy enhance immune-mediated viral control and reduce disease progression. Given its superior efficacy and similar safety profile, it should be considered a frontline option, especially for patients at higher risk of HCC or with suboptimal response to monotherapy. This systematic review provides strong evidence supporting the adoption of combination regimens in individualized Chronic Hepatitis B management.
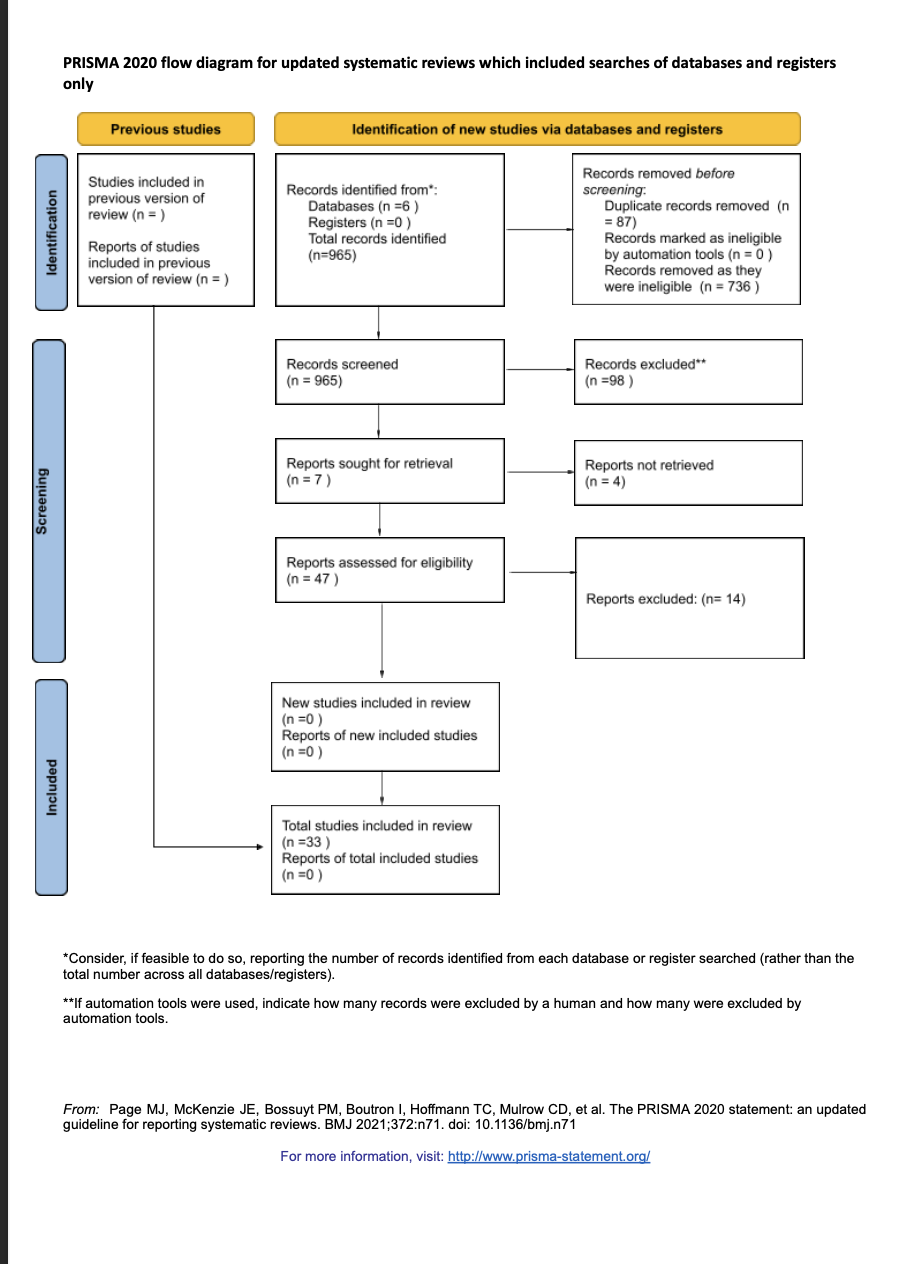
Figure: This flow diagram illustrates the study selection process. A total of 965 records were identified through database searches (PubMed, EMBASE, Cochrane). After screening and eligibility assessment, 33 studies were included in the final systematic review comparing combination vs monotherapy in chronic hepatitis B.
Disclosures:
Pinky Bai indicated no relevant financial relationships.
Ali Chand indicated no relevant financial relationships.
Aasta Kumari indicated no relevant financial relationships.
Mohammad Hassan indicated no relevant financial relationships.
Musa Khalil indicated no relevant financial relationships.
Nakul Ganju indicated no relevant financial relationships.
Sunny Kumar indicated no relevant financial relationships.
Bisma Farooq indicated no relevant financial relationships.
Angesom Kibreab indicated no relevant financial relationships.
Pinky Bai, MD1, Ali Chand, MD1, Aasta Kumari, MD2, Mohammad Hassan, MD1, Musa Khalil, MD1, Nakul Ganju, MD3, Sunny Kumar, MD4, Bisma Farooq, MBBS5, Angesom Kibreab, MD1. P1291 - Combination Therapy Improves Outcomes in Chronic Hepatitis B: A Systematic Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Howard University Hospital, Washington, DC; 2North Central Bronx Hospital, New York, NY; 3Department of Medicine, Howard University Hospital, Washington, DC; 4Wright Center for Graduate Medical Education, Scranton, PA; 5Shaikh Khalifa Bin Zayed Al Nahyan Medical and Dental Collage, Washington, DC
Introduction: Chronic Hepatitis B virus (CHB virus) affects over 250 million individuals globally and remains a leading cause of liver-related morbidity and mortality, including cirrhosis and hepatocellular carcinoma (HCC). The primary goal of antiviral treatment is sustained viral suppression to prevent disease progression. This systematic review compares the efficacy and safety of monotherapy versus combination therapy in CHB, focusing on virological and serological outcomes.
Methods: A systematic literature search was conducted using PubMed, EMBASE, and Cochrane databases for peer-reviewed studies published from 2013 to 2023. Eligible studies included randomized controlled trials (RCTs), cohort studies, and meta-analyses evaluating nucleoside/nucleotide analogues or biologics, either as monotherapy or in combination, in adult patients with CHB. Study selection followed the PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. Of 965 records screened, 33 studies met inclusion criteria. Data were extracted on HBsAg clearance, HBV DNA suppression, ALT normalization, and adverse events.
Results: Combination therapy—most commonly involving pegylated interferon (PEG-IFN) with tenofovir or entecavir—demonstrated superior efficacy compared to monotherapy. HBsAg seroclearance was observed in 12–28% of patients receiving combination therapy, compared to 2–10% in monotherapy groups. HBV DNA suppression to undetectable levels was achieved in 78–95% of patients on combination therapy, versus 60–80% on monotherapy. Combination regimens also correlated with a lower incidence of HCC over long-term follow-up. Adverse event rates were similar across both treatment groups in over 90% of studies.
Discussion: The synergistic effects of combination therapy enhance immune-mediated viral control and reduce disease progression. Given its superior efficacy and similar safety profile, it should be considered a frontline option, especially for patients at higher risk of HCC or with suboptimal response to monotherapy. This systematic review provides strong evidence supporting the adoption of combination regimens in individualized Chronic Hepatitis B management.

Figure: This flow diagram illustrates the study selection process. A total of 965 records were identified through database searches (PubMed, EMBASE, Cochrane). After screening and eligibility assessment, 33 studies were included in the final systematic review comparing combination vs monotherapy in chronic hepatitis B.
Disclosures:
Pinky Bai indicated no relevant financial relationships.
Ali Chand indicated no relevant financial relationships.
Aasta Kumari indicated no relevant financial relationships.
Mohammad Hassan indicated no relevant financial relationships.
Musa Khalil indicated no relevant financial relationships.
Nakul Ganju indicated no relevant financial relationships.
Sunny Kumar indicated no relevant financial relationships.
Bisma Farooq indicated no relevant financial relationships.
Angesom Kibreab indicated no relevant financial relationships.
Pinky Bai, MD1, Ali Chand, MD1, Aasta Kumari, MD2, Mohammad Hassan, MD1, Musa Khalil, MD1, Nakul Ganju, MD3, Sunny Kumar, MD4, Bisma Farooq, MBBS5, Angesom Kibreab, MD1. P1291 - Combination Therapy Improves Outcomes in Chronic Hepatitis B: A Systematic Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
