Sunday Poster Session
Category: IBD
P1228 - Vedolizumab as a Second Line Biologic for Cronkhite-Canada Syndrome
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- JP
Jonathan Pham, MD
University of Texas at Austin Dell Medical School
Austin, TX
Presenting Author(s)
Jonathan Pham, MD, Linda A. Feagins, MD
University of Texas at Austin Dell Medical School, Austin, TX
Introduction: Cronkhite-Canada Syndrome (CCS) is a rare, debilitating disease characterized by diffuse gastrointestinal polyposis, protein-losing enteropathy, diarrhea, and dermatologic manifestations. Nutritional support and immune modulating therapies are the mainstay of treatment. We present a case of a patient with CCS treated with vedolizumab (VDZ) to highlight an alternative treatment not discussed in the current literature.
Case Description/
Methods: A 67y/o female was diagnosed with CCS in 2020 with classic clinical symptoms and findings on endoscopy and histology. At diagnosis, her blood inflammatory markers were normal but her calprotectin was elevated (486mcg/g). She started infliximab (IFX) and azathioprine (AZA) in 10/2020 with resolution of her clinical symptoms, normalization of her calprotectin, and substantially improved but had incomplete response on repeated endoscopic and histologic evaluations. Mild residual disease was noted in the antrum. Her AZA was stopped due to abdominal pain, distension, nausea and vomiting and due to abnormal liver tests including elevated bilirubin. IFX was continued and the dosing was optimized to 10mg/kg every 8 weeks with drug levels of 13.9ug/mL. In 8/2024, she had return of her clinical symptoms, though repeat calprotectin remained normal. A repeat EGD and colonoscopy subsequently showed severe edema and nodularity of the antrum/duodenum and mild nodularity in the right colon. She started oral prednisone and initiated VDZ 11/2024. She gained weight and had improvement of her nails, hair, abdominal pain, and diarrhea. After 4 infusions of VDZ and 4 weeks off corticosteroids, a repeat EGD/colonoscopy 3/2025 showed marked improvement with only a few areas of minimal disease activity, much improved compared to the endoscopic appearance with IFX. She has continued to feel well with last follow up on 4/8/25.
Discussion: When untreated, CCS can be devastating with a 5-year mortality estimated at 55% due to complications from the disease. With immunosuppressant treatment and nutritional support, a recent case series found a 5-year overall survival at 93%. While corticosteroids can be effective for disease remission; steroid-sparing strategies are preferred. Thiopurines and IFX are well described in the literature for maintaining remission. This is the first case report to date of VDZ as an alternative therapy, particularly in a patient with secondary loss of response to IFX. This highlights an important alternative treatment option.
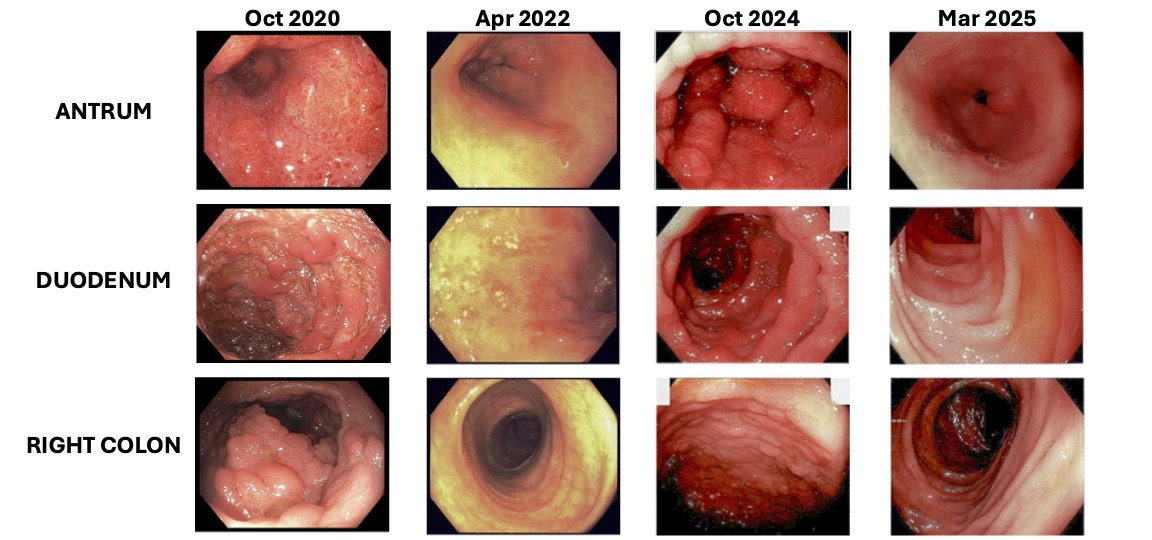
Figure: Endoscopic disease activity of the antrum, duodenum, and colon at various timepoints. October 2020: Initial diagnosis not on therapy. April 2022: IFX dose 10mg/kg, IFX level 13.9ug/mL, and IFX Ab <10ng/mL. October 2024: IFX dose 10mg/kg, IFX level 14.1ug/mL, and IFX Ab <20ng/mL. March 2025: VDZ 300mg, VDZ level 27.4mcg/mL, VDZ Ab <9.8ng/mL.
IFX: infliximab, Ab: antibody, VDZ: vedolizumab.
Disclosures:
Jonathan Pham indicated no relevant financial relationships.
Linda Feagins: Corevitas – Clinical trial participation. Takeda – Clinical trial participation.
Jonathan Pham, MD, Linda A. Feagins, MD. P1228 - Vedolizumab as a Second Line Biologic for Cronkhite-Canada Syndrome, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
University of Texas at Austin Dell Medical School, Austin, TX
Introduction: Cronkhite-Canada Syndrome (CCS) is a rare, debilitating disease characterized by diffuse gastrointestinal polyposis, protein-losing enteropathy, diarrhea, and dermatologic manifestations. Nutritional support and immune modulating therapies are the mainstay of treatment. We present a case of a patient with CCS treated with vedolizumab (VDZ) to highlight an alternative treatment not discussed in the current literature.
Case Description/
Methods: A 67y/o female was diagnosed with CCS in 2020 with classic clinical symptoms and findings on endoscopy and histology. At diagnosis, her blood inflammatory markers were normal but her calprotectin was elevated (486mcg/g). She started infliximab (IFX) and azathioprine (AZA) in 10/2020 with resolution of her clinical symptoms, normalization of her calprotectin, and substantially improved but had incomplete response on repeated endoscopic and histologic evaluations. Mild residual disease was noted in the antrum. Her AZA was stopped due to abdominal pain, distension, nausea and vomiting and due to abnormal liver tests including elevated bilirubin. IFX was continued and the dosing was optimized to 10mg/kg every 8 weeks with drug levels of 13.9ug/mL. In 8/2024, she had return of her clinical symptoms, though repeat calprotectin remained normal. A repeat EGD and colonoscopy subsequently showed severe edema and nodularity of the antrum/duodenum and mild nodularity in the right colon. She started oral prednisone and initiated VDZ 11/2024. She gained weight and had improvement of her nails, hair, abdominal pain, and diarrhea. After 4 infusions of VDZ and 4 weeks off corticosteroids, a repeat EGD/colonoscopy 3/2025 showed marked improvement with only a few areas of minimal disease activity, much improved compared to the endoscopic appearance with IFX. She has continued to feel well with last follow up on 4/8/25.
Discussion: When untreated, CCS can be devastating with a 5-year mortality estimated at 55% due to complications from the disease. With immunosuppressant treatment and nutritional support, a recent case series found a 5-year overall survival at 93%. While corticosteroids can be effective for disease remission; steroid-sparing strategies are preferred. Thiopurines and IFX are well described in the literature for maintaining remission. This is the first case report to date of VDZ as an alternative therapy, particularly in a patient with secondary loss of response to IFX. This highlights an important alternative treatment option.

Figure: Endoscopic disease activity of the antrum, duodenum, and colon at various timepoints. October 2020: Initial diagnosis not on therapy. April 2022: IFX dose 10mg/kg, IFX level 13.9ug/mL, and IFX Ab <10ng/mL. October 2024: IFX dose 10mg/kg, IFX level 14.1ug/mL, and IFX Ab <20ng/mL. March 2025: VDZ 300mg, VDZ level 27.4mcg/mL, VDZ Ab <9.8ng/mL.
IFX: infliximab, Ab: antibody, VDZ: vedolizumab.
Disclosures:
Jonathan Pham indicated no relevant financial relationships.
Linda Feagins: Corevitas – Clinical trial participation. Takeda – Clinical trial participation.
Jonathan Pham, MD, Linda A. Feagins, MD. P1228 - Vedolizumab as a Second Line Biologic for Cronkhite-Canada Syndrome, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
