Sunday Poster Session
Category: IBD
P1224 - Patient Outcomes With Intensified Risankizumab Maintenance Dosing for Crohn’s Disease: A Case Series
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Fnu Rashi, MD
Henry Ford Macomb Hospital
Clinton Township, TX
Presenting Author(s)
Award: ACG Presidential Poster Award
Fnu Rashi, MD1, Najwa El-Nachef, MD2
1Henry Ford Macomb Hospital, Clinton Township, MI; 2Henry Ford Health, Detroit, MI
Introduction: Risankizumab (RSA), an interluekin-23 inhibitor, is approved for the treatment of moderate-to-severe Crohn’s disease (CD). The standard maintenance dose of RSA is subcutaneous injection (SC) every 8 weeks. We present a series of patients with Crohn’s disease who had initial response to RSA but later required dose escalation to improve and/or recapture response to RSA.
Case Description/
Methods: Due to partial or waning response to RSA at standard dosing, five CD patients at a tertiary inflammatory bowel disease center underwent intensification of maintenance therapy to receive RSA every 4 weeks. All had ileocolonic disease; two also had perianal fistulizing CD. Two escalated due to partial response, two due to loss of response after an average of 13 months, and one had refractory disease. Patients had failed an average of two prior advanced therapies. One patient received concurrent Upadacitinib in addition to q 4 weekly RSA. Three required steroids at the time of intensification. Extraintestinal manifestations (EIM) were present in one patient.
Four of five reported clinical improvement at follow-up with a follow-up range of 6 to 14 months. The fifth patient has not had scheduled follow up since intensification of regimen. Fecal calprotectin data was available for three patients and decreased by 50% or greater in all of them. Endoscopic follow-up was available for three patients and showed improvement in each. All three patients who were on steroids at time of dose intensification were able to wean off. The only patient with EIM reported significant improvement in her arthralgia. No significant adverse safety concerns were noted with 4- weekly dosing.
Discussion: While dose escalation of other biologic therapies has been associated with improved outcomes in certain patients with CD, comparable data for RSA are currently limited. This case series supports clinical response to 4-weekly RSA in patients who relapsed or only partially improved on the q 8-weekly regimen, with no increase in adverse events. These findings encourage further exploration of intensified RSA dosing in select CD patients.
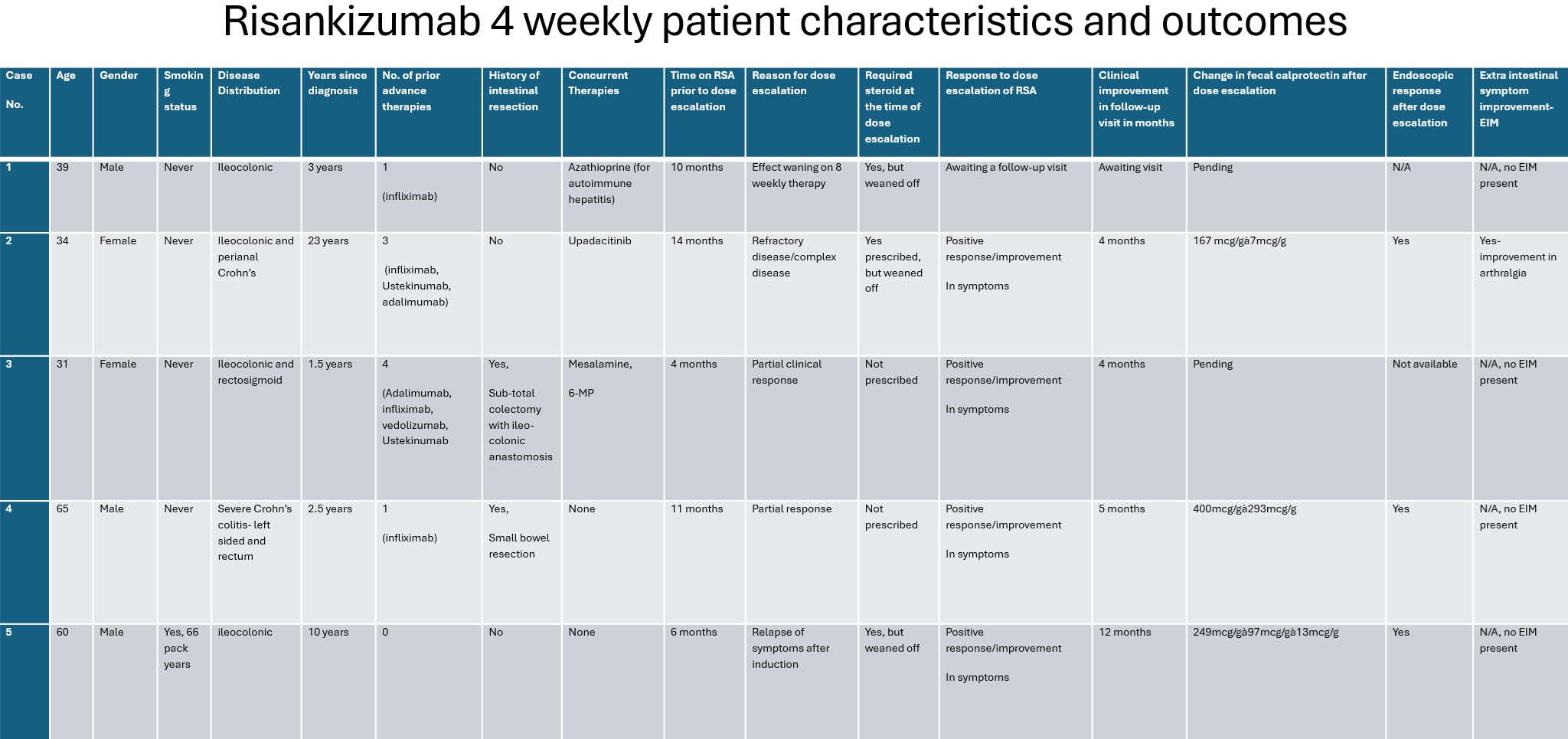
Figure: Risankizumab 4 weekly Patient Characteristics and Outcomes
Disclosures:
Fnu Rashi indicated no relevant financial relationships.
Najwa El-Nachef: Abbvie – Grant/Research Support. Abivax – Grant/Research Support. Genentech – Grant/Research Support. Takeda – Grant/Research Support.
Fnu Rashi, MD1, Najwa El-Nachef, MD2. P1224 - Patient Outcomes With Intensified Risankizumab Maintenance Dosing for Crohn’s Disease: A Case Series, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Fnu Rashi, MD1, Najwa El-Nachef, MD2
1Henry Ford Macomb Hospital, Clinton Township, MI; 2Henry Ford Health, Detroit, MI
Introduction: Risankizumab (RSA), an interluekin-23 inhibitor, is approved for the treatment of moderate-to-severe Crohn’s disease (CD). The standard maintenance dose of RSA is subcutaneous injection (SC) every 8 weeks. We present a series of patients with Crohn’s disease who had initial response to RSA but later required dose escalation to improve and/or recapture response to RSA.
Case Description/
Methods: Due to partial or waning response to RSA at standard dosing, five CD patients at a tertiary inflammatory bowel disease center underwent intensification of maintenance therapy to receive RSA every 4 weeks. All had ileocolonic disease; two also had perianal fistulizing CD. Two escalated due to partial response, two due to loss of response after an average of 13 months, and one had refractory disease. Patients had failed an average of two prior advanced therapies. One patient received concurrent Upadacitinib in addition to q 4 weekly RSA. Three required steroids at the time of intensification. Extraintestinal manifestations (EIM) were present in one patient.
Four of five reported clinical improvement at follow-up with a follow-up range of 6 to 14 months. The fifth patient has not had scheduled follow up since intensification of regimen. Fecal calprotectin data was available for three patients and decreased by 50% or greater in all of them. Endoscopic follow-up was available for three patients and showed improvement in each. All three patients who were on steroids at time of dose intensification were able to wean off. The only patient with EIM reported significant improvement in her arthralgia. No significant adverse safety concerns were noted with 4- weekly dosing.
Discussion: While dose escalation of other biologic therapies has been associated with improved outcomes in certain patients with CD, comparable data for RSA are currently limited. This case series supports clinical response to 4-weekly RSA in patients who relapsed or only partially improved on the q 8-weekly regimen, with no increase in adverse events. These findings encourage further exploration of intensified RSA dosing in select CD patients.

Figure: Risankizumab 4 weekly Patient Characteristics and Outcomes
Disclosures:
Fnu Rashi indicated no relevant financial relationships.
Najwa El-Nachef: Abbvie – Grant/Research Support. Abivax – Grant/Research Support. Genentech – Grant/Research Support. Takeda – Grant/Research Support.
Fnu Rashi, MD1, Najwa El-Nachef, MD2. P1224 - Patient Outcomes With Intensified Risankizumab Maintenance Dosing for Crohn’s Disease: A Case Series, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.


