Sunday Poster Session
Category: IBD
P1155 - Association Between SGLT2 Inhibitor Use and Inflammatory Bowel Disease in Non-Diabetic Patients
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Xiaoliang Wang, MD, PhD (he/him/his)
Cleveland Clinic
Cleveland, OH
Presenting Author(s)
Xiaoliang Wang, MD, PhD1, Darwin Tan, BS2, Manuel Braga Neto, MD, PhD1, Taha Qazi, MD3
1Cleveland Clinic, Cleveland, OH; 2Case Western Reserve University School of Medicine, Cleveland, OH; 3Cleveland Clinic Foundation, Cleveland, OH
Introduction: Sodium–glucose cotransporter-2 inhibitors (SGLT2is) are now standard treatments for chronic kidney disease (CKD) and heart failure (HF), including in patients without diabetes. In addition to lowering blood glucose, SGLT2is possess anti-inflammatory and antioxidative properties, which may contribute to their benefits in CKD and HF. Although early studies have suggested a potential protective effect of SGLT2is against Crohn’s disease in patients with type 2 diabetes, their role in preventing inflammatory bowel disease (IBD) and related complications in non-diabetic patients with CKD or HF remains unclear. This study aimed to assess whether SGLT2i use is associated with a reduced risk of IBD and its complications in this population.
Methods: We conducted a retrospective cohort study using TriNetX, a network of de-identified EHRs from inpatient and outpatient settings. Adults with CKD or HF diagnosed in the past five years were identified via ICD-10 codes. Patients with diabetes or prior IBD were excluded. The exposure group included those prescribed SGLT2is; controls had no SGLT2i use. Propensity score matching (1:1) was done using age, sex, race, ethnicity, obesity, smoking, transplant history, abdominal surgery, and selected medications (GLP-1 agonists, DPP-4 inhibitors, ACE inhibitors, ARBs, beta-blockers, spironolactone). We assessed incidence of different type of UC and CD, reported as ORs with 95% CIs.
Results: After 1:1 propensity score matching, each cohort included 119,250 non-diabetic patients with CKD or HF. SGLT2i users showed significantly lower odds of developing ulcerative colitis (UC) (OR 0.62; 95% CI, 0.54–0.70) and Crohn’s disease (CD) (OR 0.74; 95% CI, 0.62–0.88) compared to controls. Subtype analysis revealed reduced odds for ulcerative pancolitis (OR 0.54; 95% CI, 0.40–0.72), ulcerative proctitis (OR 0.56; 95% CI, 0.36–0.87), left-sided colitis (OR 0.41; 95% CI, 0.21–0.77), CD of the large intestine (OR 0.66; 95% CI, 0.47–0.92), and CD involving both small and large intestines (OR 0.61; 95% CI, 0.40–0.94).
Discussion: In this large, propensity-matched cohort of non-diabetic patients with CKD or HF, SGLT2i use was associated with a significantly reduced risk of developing ulcerative colitis or Crohn’s disease. These findings suggest a potential gastrointestinal protective effect of SGLT2 inhibitors and further studies are needed to validate these results and further investigate the underlying mechanisms.
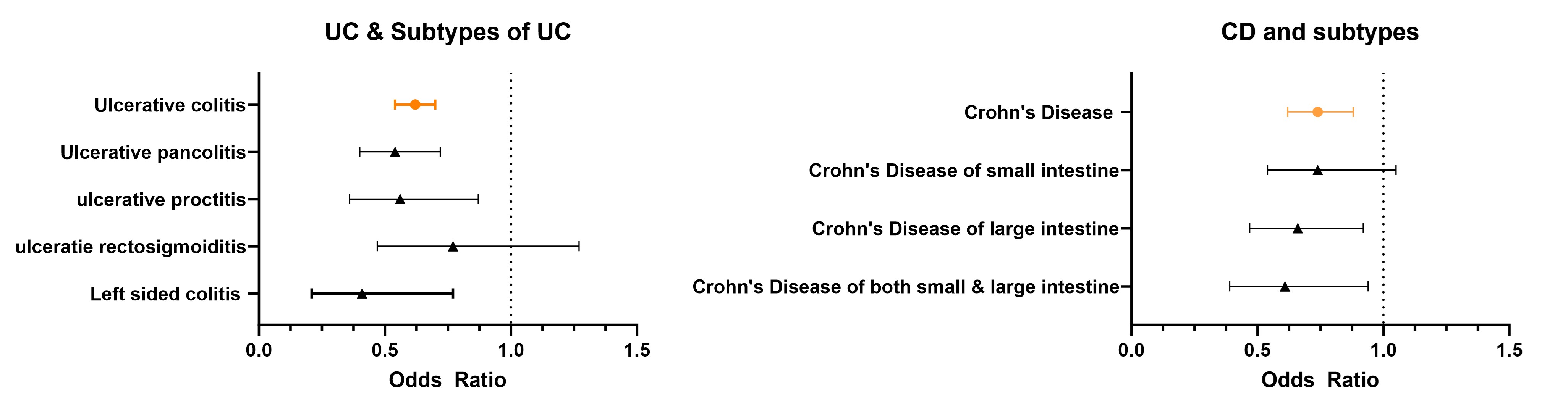
Figure: Odds ratio of different type of UC/CD in non-diabetic patients on SGLT2i after Propensity matching.
Disclosures:
Xiaoliang Wang indicated no relevant financial relationships.
Darwin Tan indicated no relevant financial relationships.
Manuel Braga Neto indicated no relevant financial relationships.
Taha Qazi: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Celltirion – Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Johnson and Johnson – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. pfizer – Grant/Research Support.
Xiaoliang Wang, MD, PhD1, Darwin Tan, BS2, Manuel Braga Neto, MD, PhD1, Taha Qazi, MD3. P1155 - Association Between SGLT2 Inhibitor Use and Inflammatory Bowel Disease in Non-Diabetic Patients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Cleveland Clinic, Cleveland, OH; 2Case Western Reserve University School of Medicine, Cleveland, OH; 3Cleveland Clinic Foundation, Cleveland, OH
Introduction: Sodium–glucose cotransporter-2 inhibitors (SGLT2is) are now standard treatments for chronic kidney disease (CKD) and heart failure (HF), including in patients without diabetes. In addition to lowering blood glucose, SGLT2is possess anti-inflammatory and antioxidative properties, which may contribute to their benefits in CKD and HF. Although early studies have suggested a potential protective effect of SGLT2is against Crohn’s disease in patients with type 2 diabetes, their role in preventing inflammatory bowel disease (IBD) and related complications in non-diabetic patients with CKD or HF remains unclear. This study aimed to assess whether SGLT2i use is associated with a reduced risk of IBD and its complications in this population.
Methods: We conducted a retrospective cohort study using TriNetX, a network of de-identified EHRs from inpatient and outpatient settings. Adults with CKD or HF diagnosed in the past five years were identified via ICD-10 codes. Patients with diabetes or prior IBD were excluded. The exposure group included those prescribed SGLT2is; controls had no SGLT2i use. Propensity score matching (1:1) was done using age, sex, race, ethnicity, obesity, smoking, transplant history, abdominal surgery, and selected medications (GLP-1 agonists, DPP-4 inhibitors, ACE inhibitors, ARBs, beta-blockers, spironolactone). We assessed incidence of different type of UC and CD, reported as ORs with 95% CIs.
Results: After 1:1 propensity score matching, each cohort included 119,250 non-diabetic patients with CKD or HF. SGLT2i users showed significantly lower odds of developing ulcerative colitis (UC) (OR 0.62; 95% CI, 0.54–0.70) and Crohn’s disease (CD) (OR 0.74; 95% CI, 0.62–0.88) compared to controls. Subtype analysis revealed reduced odds for ulcerative pancolitis (OR 0.54; 95% CI, 0.40–0.72), ulcerative proctitis (OR 0.56; 95% CI, 0.36–0.87), left-sided colitis (OR 0.41; 95% CI, 0.21–0.77), CD of the large intestine (OR 0.66; 95% CI, 0.47–0.92), and CD involving both small and large intestines (OR 0.61; 95% CI, 0.40–0.94).
Discussion: In this large, propensity-matched cohort of non-diabetic patients with CKD or HF, SGLT2i use was associated with a significantly reduced risk of developing ulcerative colitis or Crohn’s disease. These findings suggest a potential gastrointestinal protective effect of SGLT2 inhibitors and further studies are needed to validate these results and further investigate the underlying mechanisms.

Figure: Odds ratio of different type of UC/CD in non-diabetic patients on SGLT2i after Propensity matching.
Disclosures:
Xiaoliang Wang indicated no relevant financial relationships.
Darwin Tan indicated no relevant financial relationships.
Manuel Braga Neto indicated no relevant financial relationships.
Taha Qazi: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Celltirion – Grant/Research Support. Eli Lilly – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Johnson and Johnson – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. pfizer – Grant/Research Support.
Xiaoliang Wang, MD, PhD1, Darwin Tan, BS2, Manuel Braga Neto, MD, PhD1, Taha Qazi, MD3. P1155 - Association Between SGLT2 Inhibitor Use and Inflammatory Bowel Disease in Non-Diabetic Patients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
