Sunday Poster Session
Category: IBD
P1149 - Mirikizumab Sustains Improvement in Fatigue, Abdominal Pain, and Stool Frequency Following 104 Weeks of Continuous Treatment for Crohn’s Disease: Results From the VIVID-2 Open-Label Extension Study
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Jessica R. Allegretti, MD, MPH, FACG (she/her/hers)
Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School
Boston, MA
Presenting Author(s)
Peter Bossuyt, 1, Ashwin Ananthakrishnan, 2, Aisha Vadhariya, 3, Na Lu, 4, Guanglei Yu, PhD5, Jianmin Wu, 3, Jessica R.. Allegretti, MD, MPH6, Minhu Chen, MD, PhD7
1Imelda General Hospital, Bonheiden, Antwerpen, Belgium; 2Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA; 3Eli Lilly and Company, Indianapolis, IN; 4Precision Statistics Consulting Inc, Woodbury, Woodbury, MN; 5Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 6Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 7The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
Introduction: Mirikizumab (MIRI), a p19-directed antibody against interleukin-23, demonstrated improvements in fatigue, abdominal pain (AP) and stool frequency (SF) at Week (W) 12 and W52 in the VIVID-1 clinical trial.
Methods: This analysis shows the effect of MIRI on fatigue, AP, SF and achievement of clinical remission by Patient-Reported Outcome (PRO; SF ≤3 and AP ≤1 and neither worse than baseline) in the VIVID-2 open-label extension study through W104. This analysis includes data from primary analysis set in patients with Crohn’s disease (CD) randomized to the MIRI treatment arm in VIVID-1 who achieved endoscopic response (≥50% reduction from baseline in Simple endoscopic score for CD) at W52 and continued the MIRI subcutaneous dosing (300 mg) in VIVID-2. Change from VIVID-1 baseline was measured for continuous endpoints. Endpoints: fatigue measured using FACIT-Fatigue (measured as change from baseline, and clinically-meaningful improvement of ≥6-points among patients with baseline ≤46), AP improvement (measured as change from baseline, and achievement of ≤1 among patients with baseline >1), SF improvement (measured as change from baseline, and achievement of ≤3 among patients with baseline >3) and clinical remission by PRO, assessed at W64 and 104. Missing data were handled using observed case (OC) and modified non-responder imputation (mNRI) for response rates, while using modified baseline observation carried forward (mBOCF) for change from baseline. Response rates were presented as unadjusted proportions with 2-sided 95% confidence intervals using the Rubin's Rules (mNRI). ANCOVA models were used for continuous variables, adjusting for baseline values and intervention group.
Results: Fatigue mean (standard error [SE]) change from baseline was 10.7 (0.56) at W64; 9.4 (0.59) at W104, (observed response, n [%]: 152 [67.6%] at W64; 136 [64.8%] at W104; Table). AP improved (mean [SE] change: −1.5 [0.04] at W64; −1.6 [0.05] at W104), with response rates increasing from 174 (77.3%) to 161 (84.7%). SF showed sustained improvement (mean [SE] change: −4.3 [0.14] at W64; −4.3 [0.16] at W104), with response rates reaching 155 (89.6%) at W104. Similar rates of achievement of clinical remission by PRO were observed (178 [73.6%] at W64; 165 [80.1%] at W104) (Table).
Discussion: MIRI sustained improvement in fatigue, AP, SF and clinical remission by PRO through 104 weeks of continuous treatment. These consistent findings support the long-term treatment with MIRI to address key symptoms of CD.
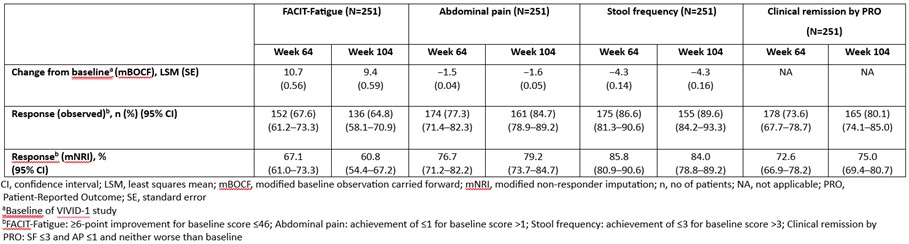
Figure: Improvement in Fatigue, Abdominal Pain, Stool Frequency and Achievement of Clinical Remission by PRO up to Week 104
Disclosures:
Peter Bossuyt: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Lecture fees. Amgen – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Lecture fees. CAG – Lecture fees. Celltrion – Advisory Committee/Board Member, Lecture fees. CIRC – Advisory Committee/Board Member. Dr. Falk Pharma Benelux – Advisory Committee/Board Member. Eli Lilly and Company – Advisory Committee/Board Member, Lecture fees. EPGS – Lecture fees. Galapagos NV – Advisory Committee/Board Member, Lecture fees. Globalport – Lecture fees. Janssen – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Lecture fees. Janssen – Lecture fees. Materia Prima – Lecture fees. Pentax – Advisory Committee/Board Member, Lecture fees. Pfizer – Grant/Research Support. PSI-CRO – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member. Scope – Lecture fees. Takeda – Advisory Committee/Board Member, Lecture fees. Tetrameros – Advisory Committee/Board Member. Viatris – Grant/Research Support.
Ashwin Ananthakrishnan: Takeda – Grant/Research Support.
Aisha Vadhariya: Eli Lilly and Company – Employee, Stock Options.
Na Lu: Precision Statistics Consulting – Employee.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Jianmin Wu: Eli Lilly and Company – Employee, Stock Options.
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Minhu Chen: AbbVie – Provided Educational Activities. Boehringer Ingelheim – Advisory Committee/Board Member. China Medical System – Provided Educational Activities. IPSEN – Provided Educational Activities. Johnson & Johnson – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Takeda – Grant/Research Support, Provided Educational Activities.
Peter Bossuyt, 1, Ashwin Ananthakrishnan, 2, Aisha Vadhariya, 3, Na Lu, 4, Guanglei Yu, PhD5, Jianmin Wu, 3, Jessica R.. Allegretti, MD, MPH6, Minhu Chen, MD, PhD7. P1149 - Mirikizumab Sustains Improvement in Fatigue, Abdominal Pain, and Stool Frequency Following 104 Weeks of Continuous Treatment for Crohn’s Disease: Results From the VIVID-2 Open-Label Extension Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Imelda General Hospital, Bonheiden, Antwerpen, Belgium; 2Division of Gastroenterology, Massachusetts General Hospital and Harvard Medical School, Boston, MA; 3Eli Lilly and Company, Indianapolis, IN; 4Precision Statistics Consulting Inc, Woodbury, Woodbury, MN; 5Eli Lilly and Company, Indianapolis, Indiana, USA, Indianapolis, IN; 6Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 7The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, Guangdong, China
Introduction: Mirikizumab (MIRI), a p19-directed antibody against interleukin-23, demonstrated improvements in fatigue, abdominal pain (AP) and stool frequency (SF) at Week (W) 12 and W52 in the VIVID-1 clinical trial.
Methods: This analysis shows the effect of MIRI on fatigue, AP, SF and achievement of clinical remission by Patient-Reported Outcome (PRO; SF ≤3 and AP ≤1 and neither worse than baseline) in the VIVID-2 open-label extension study through W104. This analysis includes data from primary analysis set in patients with Crohn’s disease (CD) randomized to the MIRI treatment arm in VIVID-1 who achieved endoscopic response (≥50% reduction from baseline in Simple endoscopic score for CD) at W52 and continued the MIRI subcutaneous dosing (300 mg) in VIVID-2. Change from VIVID-1 baseline was measured for continuous endpoints. Endpoints: fatigue measured using FACIT-Fatigue (measured as change from baseline, and clinically-meaningful improvement of ≥6-points among patients with baseline ≤46), AP improvement (measured as change from baseline, and achievement of ≤1 among patients with baseline >1), SF improvement (measured as change from baseline, and achievement of ≤3 among patients with baseline >3) and clinical remission by PRO, assessed at W64 and 104. Missing data were handled using observed case (OC) and modified non-responder imputation (mNRI) for response rates, while using modified baseline observation carried forward (mBOCF) for change from baseline. Response rates were presented as unadjusted proportions with 2-sided 95% confidence intervals using the Rubin's Rules (mNRI). ANCOVA models were used for continuous variables, adjusting for baseline values and intervention group.
Results: Fatigue mean (standard error [SE]) change from baseline was 10.7 (0.56) at W64; 9.4 (0.59) at W104, (observed response, n [%]: 152 [67.6%] at W64; 136 [64.8%] at W104; Table). AP improved (mean [SE] change: −1.5 [0.04] at W64; −1.6 [0.05] at W104), with response rates increasing from 174 (77.3%) to 161 (84.7%). SF showed sustained improvement (mean [SE] change: −4.3 [0.14] at W64; −4.3 [0.16] at W104), with response rates reaching 155 (89.6%) at W104. Similar rates of achievement of clinical remission by PRO were observed (178 [73.6%] at W64; 165 [80.1%] at W104) (Table).
Discussion: MIRI sustained improvement in fatigue, AP, SF and clinical remission by PRO through 104 weeks of continuous treatment. These consistent findings support the long-term treatment with MIRI to address key symptoms of CD.

Figure: Improvement in Fatigue, Abdominal Pain, Stool Frequency and Achievement of Clinical Remission by PRO up to Week 104
Disclosures:
Peter Bossuyt: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Lecture fees. Amgen – Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Lecture fees. CAG – Lecture fees. Celltrion – Advisory Committee/Board Member, Lecture fees. CIRC – Advisory Committee/Board Member. Dr. Falk Pharma Benelux – Advisory Committee/Board Member. Eli Lilly and Company – Advisory Committee/Board Member, Lecture fees. EPGS – Lecture fees. Galapagos NV – Advisory Committee/Board Member, Lecture fees. Globalport – Lecture fees. Janssen – Advisory Committee/Board Member. Janssen – Advisory Committee/Board Member, Grant/Research Support, Lecture fees. Janssen – Lecture fees. Materia Prima – Lecture fees. Pentax – Advisory Committee/Board Member, Lecture fees. Pfizer – Grant/Research Support. PSI-CRO – Advisory Committee/Board Member. Roche – Advisory Committee/Board Member. Scope – Lecture fees. Takeda – Advisory Committee/Board Member, Lecture fees. Tetrameros – Advisory Committee/Board Member. Viatris – Grant/Research Support.
Ashwin Ananthakrishnan: Takeda – Grant/Research Support.
Aisha Vadhariya: Eli Lilly and Company – Employee, Stock Options.
Na Lu: Precision Statistics Consulting – Employee.
Guanglei Yu: Eli Lilly and Company – Employee, Stock Options.
Jianmin Wu: Eli Lilly and Company – Employee, Stock Options.
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Minhu Chen: AbbVie – Provided Educational Activities. Boehringer Ingelheim – Advisory Committee/Board Member. China Medical System – Provided Educational Activities. IPSEN – Provided Educational Activities. Johnson & Johnson – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Takeda – Grant/Research Support, Provided Educational Activities.
Peter Bossuyt, 1, Ashwin Ananthakrishnan, 2, Aisha Vadhariya, 3, Na Lu, 4, Guanglei Yu, PhD5, Jianmin Wu, 3, Jessica R.. Allegretti, MD, MPH6, Minhu Chen, MD, PhD7. P1149 - Mirikizumab Sustains Improvement in Fatigue, Abdominal Pain, and Stool Frequency Following 104 Weeks of Continuous Treatment for Crohn’s Disease: Results From the VIVID-2 Open-Label Extension Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
