Sunday Poster Session
Category: IBD
P1141 - Potential Role of Biomarkers in Approved and Investigational Drugs for Ulcerative Colitis: Step Towards Personalized Medicine
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Akanksha Togra, MD (she/her/hers)
Texas Tech University Health Sciences Center, El Paso
El Paso, TX
Presenting Author(s)
Akanksha Togra, MD1, Aishwarya Thakurdesai, MD2, Neil Sheth, MD3, Marc J. Zuckerman, MD4, Bincy Abraham, MD, MS, FACG5
1Texas Tech University Health Sciences Center, El Paso, El Paso, TX; 2University of Louisville School of Medicine, Houston, TX; 3McGovern Medical School at UTHealth, Houston, TX; 4Division of Gastroenterology, Department of Internal Medicine, Texas Tech University Health Sciences Center, El Paso, TX., El Paso, TX; 5Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX
Introduction: Pharmacotherapies for ulcerative colitis (UC) have recently expanded, yet many patients remain unresponsive to therapy. Further, the disease course varies considerably and predicting patient response is challenging. We aim to analyze trial designs of approved and investigational drugs along with literature on personalized medicine (PM) to explore role of predictive tools such as biomarkers in treatment response assessment.
Methods: Data was extracted from three sources: Food and Drug Administration (FDA) package inserts, ClinicalTrials.gov (CTG), and PubMed. FDA labels were reviewed to evaluate trial designs of approved drugs since 2005 and CTG for trials of investigational drugs. PubMed search was conducted using terms “ulcerative colitis” AND “personalized medicine”.
Results: We evaluated 15 PM studies in UC, of which 5 had predictive biomarker assessments (Table 1). Two of these studies identified the role of gene expression. For mirikizumab, significant reduction in transcripts (drug resistance signature) of TNF receptor was noted; while for golimumab, patients with identified gene expression signature had mucosal healing at week 6 AUCROC 0.69 (P=0.002). Another study identified 37 serum proteins that significantly changed at week 8 (P< 0.05) to predict clinical efficacy to ritlecitinib. Clinical decision support tool (VDZ-CDST) was also developed to identify patients most appropriate for vedolizumab (AUC=0.72, 95% CI 0.64-0.79).
Next, we reviewed trial designs of both approved and investigational drugs. Study designs of initially approved drugs targeted patients unresponsive to conventional therapies, and later expanded to include those failing biologics and newer drugs. On CTG review of drug development efforts, we found 56 ongoing phase III clinical trials registered for UC treatment, of which only 20 were addressing PM. It was noted that while early phase studies suggested a potential for biomarkers, none of the subsequent phase 3 trials of approved or investigational drugs utilized them to predict clinical response or further evaluate their utility.
Discussion: We found that multiple early phase studies are suggesting potential of serum markers, gene expression or CDST in predicting drug response. Though a growing number of therapies have been approved, there were no predictive biomarker driven response assessments in their phase 3 trials. There is need for more research on biomarkers to support both drug development and PM decisions to eventually translate it to clinical use.
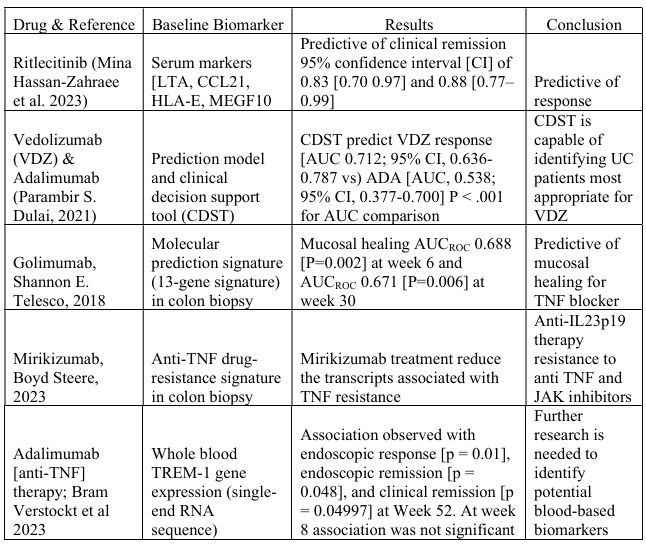
Figure: Table 1: Biomarkers evaluated in early phase trials of ulcerative colitis
Disclosures:
Akanksha Togra indicated no relevant financial relationships.
Aishwarya Thakurdesai indicated no relevant financial relationships.
Neil Sheth indicated no relevant financial relationships.
Marc Zuckerman indicated no relevant financial relationships.
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Akanksha Togra, MD1, Aishwarya Thakurdesai, MD2, Neil Sheth, MD3, Marc J. Zuckerman, MD4, Bincy Abraham, MD, MS, FACG5. P1141 - Potential Role of Biomarkers in Approved and Investigational Drugs for Ulcerative Colitis: Step Towards Personalized Medicine, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Texas Tech University Health Sciences Center, El Paso, El Paso, TX; 2University of Louisville School of Medicine, Houston, TX; 3McGovern Medical School at UTHealth, Houston, TX; 4Division of Gastroenterology, Department of Internal Medicine, Texas Tech University Health Sciences Center, El Paso, TX., El Paso, TX; 5Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX
Introduction: Pharmacotherapies for ulcerative colitis (UC) have recently expanded, yet many patients remain unresponsive to therapy. Further, the disease course varies considerably and predicting patient response is challenging. We aim to analyze trial designs of approved and investigational drugs along with literature on personalized medicine (PM) to explore role of predictive tools such as biomarkers in treatment response assessment.
Methods: Data was extracted from three sources: Food and Drug Administration (FDA) package inserts, ClinicalTrials.gov (CTG), and PubMed. FDA labels were reviewed to evaluate trial designs of approved drugs since 2005 and CTG for trials of investigational drugs. PubMed search was conducted using terms “ulcerative colitis” AND “personalized medicine”.
Results: We evaluated 15 PM studies in UC, of which 5 had predictive biomarker assessments (Table 1). Two of these studies identified the role of gene expression. For mirikizumab, significant reduction in transcripts (drug resistance signature) of TNF receptor was noted; while for golimumab, patients with identified gene expression signature had mucosal healing at week 6 AUCROC 0.69 (P=0.002). Another study identified 37 serum proteins that significantly changed at week 8 (P< 0.05) to predict clinical efficacy to ritlecitinib. Clinical decision support tool (VDZ-CDST) was also developed to identify patients most appropriate for vedolizumab (AUC=0.72, 95% CI 0.64-0.79).
Next, we reviewed trial designs of both approved and investigational drugs. Study designs of initially approved drugs targeted patients unresponsive to conventional therapies, and later expanded to include those failing biologics and newer drugs. On CTG review of drug development efforts, we found 56 ongoing phase III clinical trials registered for UC treatment, of which only 20 were addressing PM. It was noted that while early phase studies suggested a potential for biomarkers, none of the subsequent phase 3 trials of approved or investigational drugs utilized them to predict clinical response or further evaluate their utility.
Discussion: We found that multiple early phase studies are suggesting potential of serum markers, gene expression or CDST in predicting drug response. Though a growing number of therapies have been approved, there were no predictive biomarker driven response assessments in their phase 3 trials. There is need for more research on biomarkers to support both drug development and PM decisions to eventually translate it to clinical use.

Figure: Table 1: Biomarkers evaluated in early phase trials of ulcerative colitis
Disclosures:
Akanksha Togra indicated no relevant financial relationships.
Aishwarya Thakurdesai indicated no relevant financial relationships.
Neil Sheth indicated no relevant financial relationships.
Marc Zuckerman indicated no relevant financial relationships.
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Akanksha Togra, MD1, Aishwarya Thakurdesai, MD2, Neil Sheth, MD3, Marc J. Zuckerman, MD4, Bincy Abraham, MD, MS, FACG5. P1141 - Potential Role of Biomarkers in Approved and Investigational Drugs for Ulcerative Colitis: Step Towards Personalized Medicine, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
