Sunday Poster Session
Category: IBD
P1140 - Potential Role of Biomarkers in Approved and Investigational Drugs for Crohn’s Disease: Step Towards Personalized Medicine
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Akanksha Togra, MD (she/her/hers)
Texas Tech University Health Sciences Center, El Paso
El Paso, TX
Presenting Author(s)
Akanksha Togra, MD1, Aastha Bharwad, MD2, Neil Sheth, MD3, Alejandro Robles, MD4, Erik Askenasy, MD3, Nirav Thosani, MD5, Andrew Dupont, MD6
1Texas Tech University Health Sciences Center, El Paso, El Paso, TX; 2University of Texas at Houston, Houston, TX; 3McGovern Medical School at UTHealth, Houston, TX; 4Department of Gastroenterology, Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center El Paso, El Paso , TX, El Paso, TX; 5University of Texas Health, McGovern Medical School, Houston, TX; 6University of Texas Health Sciences Center in Houston, Houston, TX
Introduction: Crohn’s disease (CD) can affect any segment of the gastrointestinal tract and has an unpredictable disease course. Further, the clinical trajectory of CD varies widely, and monitoring often requires invasive tests. We aim to analyze trial designs of approved and investigational drugs along with literature on personalized medicine (PM) to explore role of predictive tools such as biomarkers in treatment response assessment.
Methods: We extracted CD treatment-related data from three sources: Food and Drug Administration (FDA) package inserts, ClinicalTrials.gov (CTG), and PubMed. FDA labels were reviewed to evaluate trial designs of approved drugs since 1998 and CTG for ongoing phase III trials of investigational drugs. PubMed search was conducted using terms “Crohn’s disease” AND “personalized medicine”.
Results: We evaluated 18 PM studies in CD, of which 4 had predictive biomarker assessments (Table 1). Two of these studies assessed inflammatory markers i.e. Brazikumab showing higher response in patients with increased IL 22 (51% vs 42%) or CRP (21% vs 18%), and Adalimumab showing increased response in higher mTNF(+) immune cells (92% vs 15%). Response to Adalimumab was also evaluated with TREM-1 expression, however this was not predictive of endoscopic (p=0.53) or clinical (p=0.79) remission. A recent study found ileal microvillar length ( >1.7µm) to be predictive of response to both vedolizumab and ustekinumab (p=0.02).
Next, we reviewed CD trial designs of both approved and investigational drugs. Study designs of initially approved drugs targeted patients unresponsive to conventional therapies, and later expanded to include those failing biologics. On CTG review of drug development efforts, we found 46 ongoing phase III clinical trials registered for CD treatment, of which 15 were addressing PM. It was noted that while translational studies suggested a potential for biomarkers, none of the subsequent phase 3 trials of approved or investigational drugs utilized them to predict clinical response or further assess their utility.
Discussion: We found that multiple translational studies are suggesting potential of inflammatory markers, gene expression and even ileal microvillar length in predicting drug response in CD. Though a growing number of therapies have been approved, there were no CD predictive biomarker driven response assessments in phase 3 trials. There is need for more research on biomarkers in CD drug development to improve personalized treatment decisions and clinical outcomes.
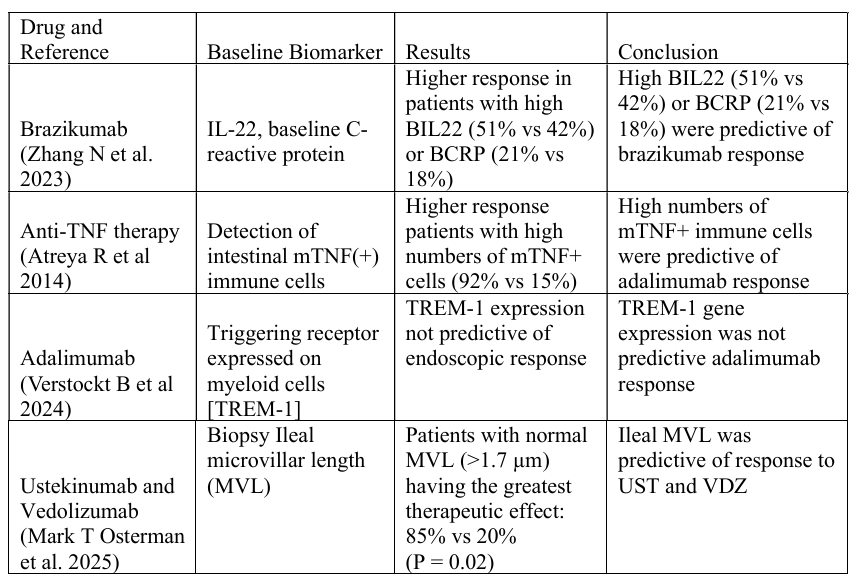
Figure: Table 1: Predictive Biomarkers in moderate to severe CD
Disclosures:
Akanksha Togra indicated no relevant financial relationships.
Aastha Bharwad indicated no relevant financial relationships.
Neil Sheth indicated no relevant financial relationships.
Alejandro Robles indicated no relevant financial relationships.
Erik Askenasy indicated no relevant financial relationships.
Nirav Thosani: Alpfa medical – Consultant. Roseaid – Creatorship rights.
Andrew Dupont indicated no relevant financial relationships.
Akanksha Togra, MD1, Aastha Bharwad, MD2, Neil Sheth, MD3, Alejandro Robles, MD4, Erik Askenasy, MD3, Nirav Thosani, MD5, Andrew Dupont, MD6. P1140 - Potential Role of Biomarkers in Approved and Investigational Drugs for Crohn’s Disease: Step Towards Personalized Medicine, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Texas Tech University Health Sciences Center, El Paso, El Paso, TX; 2University of Texas at Houston, Houston, TX; 3McGovern Medical School at UTHealth, Houston, TX; 4Department of Gastroenterology, Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center El Paso, El Paso , TX, El Paso, TX; 5University of Texas Health, McGovern Medical School, Houston, TX; 6University of Texas Health Sciences Center in Houston, Houston, TX
Introduction: Crohn’s disease (CD) can affect any segment of the gastrointestinal tract and has an unpredictable disease course. Further, the clinical trajectory of CD varies widely, and monitoring often requires invasive tests. We aim to analyze trial designs of approved and investigational drugs along with literature on personalized medicine (PM) to explore role of predictive tools such as biomarkers in treatment response assessment.
Methods: We extracted CD treatment-related data from three sources: Food and Drug Administration (FDA) package inserts, ClinicalTrials.gov (CTG), and PubMed. FDA labels were reviewed to evaluate trial designs of approved drugs since 1998 and CTG for ongoing phase III trials of investigational drugs. PubMed search was conducted using terms “Crohn’s disease” AND “personalized medicine”.
Results: We evaluated 18 PM studies in CD, of which 4 had predictive biomarker assessments (Table 1). Two of these studies assessed inflammatory markers i.e. Brazikumab showing higher response in patients with increased IL 22 (51% vs 42%) or CRP (21% vs 18%), and Adalimumab showing increased response in higher mTNF(+) immune cells (92% vs 15%). Response to Adalimumab was also evaluated with TREM-1 expression, however this was not predictive of endoscopic (p=0.53) or clinical (p=0.79) remission. A recent study found ileal microvillar length ( >1.7µm) to be predictive of response to both vedolizumab and ustekinumab (p=0.02).
Next, we reviewed CD trial designs of both approved and investigational drugs. Study designs of initially approved drugs targeted patients unresponsive to conventional therapies, and later expanded to include those failing biologics. On CTG review of drug development efforts, we found 46 ongoing phase III clinical trials registered for CD treatment, of which 15 were addressing PM. It was noted that while translational studies suggested a potential for biomarkers, none of the subsequent phase 3 trials of approved or investigational drugs utilized them to predict clinical response or further assess their utility.
Discussion: We found that multiple translational studies are suggesting potential of inflammatory markers, gene expression and even ileal microvillar length in predicting drug response in CD. Though a growing number of therapies have been approved, there were no CD predictive biomarker driven response assessments in phase 3 trials. There is need for more research on biomarkers in CD drug development to improve personalized treatment decisions and clinical outcomes.

Figure: Table 1: Predictive Biomarkers in moderate to severe CD
Disclosures:
Akanksha Togra indicated no relevant financial relationships.
Aastha Bharwad indicated no relevant financial relationships.
Neil Sheth indicated no relevant financial relationships.
Alejandro Robles indicated no relevant financial relationships.
Erik Askenasy indicated no relevant financial relationships.
Nirav Thosani: Alpfa medical – Consultant. Roseaid – Creatorship rights.
Andrew Dupont indicated no relevant financial relationships.
Akanksha Togra, MD1, Aastha Bharwad, MD2, Neil Sheth, MD3, Alejandro Robles, MD4, Erik Askenasy, MD3, Nirav Thosani, MD5, Andrew Dupont, MD6. P1140 - Potential Role of Biomarkers in Approved and Investigational Drugs for Crohn’s Disease: Step Towards Personalized Medicine, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
