Sunday Poster Session
Category: IBD
P1138 - Role of Nitroimidazoles in Crohn's Disease Recurrence in Patients Undergoing Ileocolonic Resection: A Systematic Review and Meta-Analysis
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall

Aamir Saeed, MD
Vanderbilt University Medical Center
Memphis, TN
Presenting Author(s)
Aamir Saeed, MD1, Saira Yousuf, MD1, James Salughter, PhD1, Aimal Khan, MD1, Alexander Hawkins, MD1, David A. Schwartz, MD2, Sara Horst, MD, MPH, FACG3, Baldeep Pabla, MD, MSCI2
1Vanderbilt University Medical Center, Nashville, TN; 2Vanderbilt Inflammatory Bowel Disease Clinic, Nashville, TN; 3Vanderbilt University School of Medicine, Nashville, TN
Introduction: Previous studies have shown mixed results regarding the efficacy of nitroimidazoles in preventing postoperative recurrence of CD. There is limited data to evaluate the efficacy of nitroimidazoles in post-operative recurrence rates in the era of increased biologic or advanced therapy use in the postoperative period for patients with inflammatory bowel disease. We aim to assess the endoscopic and clinical recurrence with the use of of nitroimidazole (metronidazole or ornidazole) in patients with CD undergoing ICR.
Methods: PubMed, Web of Science Core Collection, Embase, and the Cochrane Database of Systematic Reviews were reviewed to identify studies comparing outcomes of nitroimidazoles and placebo in CD recurrence in patients undergoing an ICR from inception until October 20, 2024. The primary outcome was endoscopic recurrence. The secondary outcomes included clinical recurrence and adverse events. We used the Mantel-Haenszel method to pool data for primary and secondary outcomes into random-effect model meta-analyses, we calculated pooled risk ratios (RRs), and their corresponding 95% confidence intervals (CIs) for all dichotomous variables, and the heterogeneity was assessed using the I2 statistic.
Results: Five studies (4 RCTS, 1 retrospective) comprising 432 patients (nitroimidazoles 250 and placebo 182) met inclusion criteria. The mean age was 35.2 years and 62% were male. Nitroimidazoles were associated with lower rates of endoscopic recurrence as compared to placebo at 12 months, 44% vs 66.5%, RR (95% CI): 0.68 (0.57, 0.81), p< 0.0001. Pooled rates for clinical recurrence were 18% and 31% between the groups, nitroimidazoles were associated with lower rates of clinical recurrence as compared to the placebo RR (95% CI): 0.42 (0.21, 0.86), p=0.02 (Figure 2). Nitroimidazoles were associated with lower adverse events as compared to the control group, with risk difference (95% CI): 0.20 (0.01, 0.39), p=0.04.
We also performed subgroup analysis among patients on metronidazole therapy vs placebo and 4 studies reported data on it. We found that metronidazole was associated with lower rates of endoscopic recurrence RR (95% CI): 0.67 (0.54, 0.84), p=0.0004 (Figure 1).
Discussion: Our findings observe that nitroimidazoles are associated with lower rates of endoscopic and clinical recurrence and adverse events as compared to placebo in CD recurrence in patients undergoing ICR.
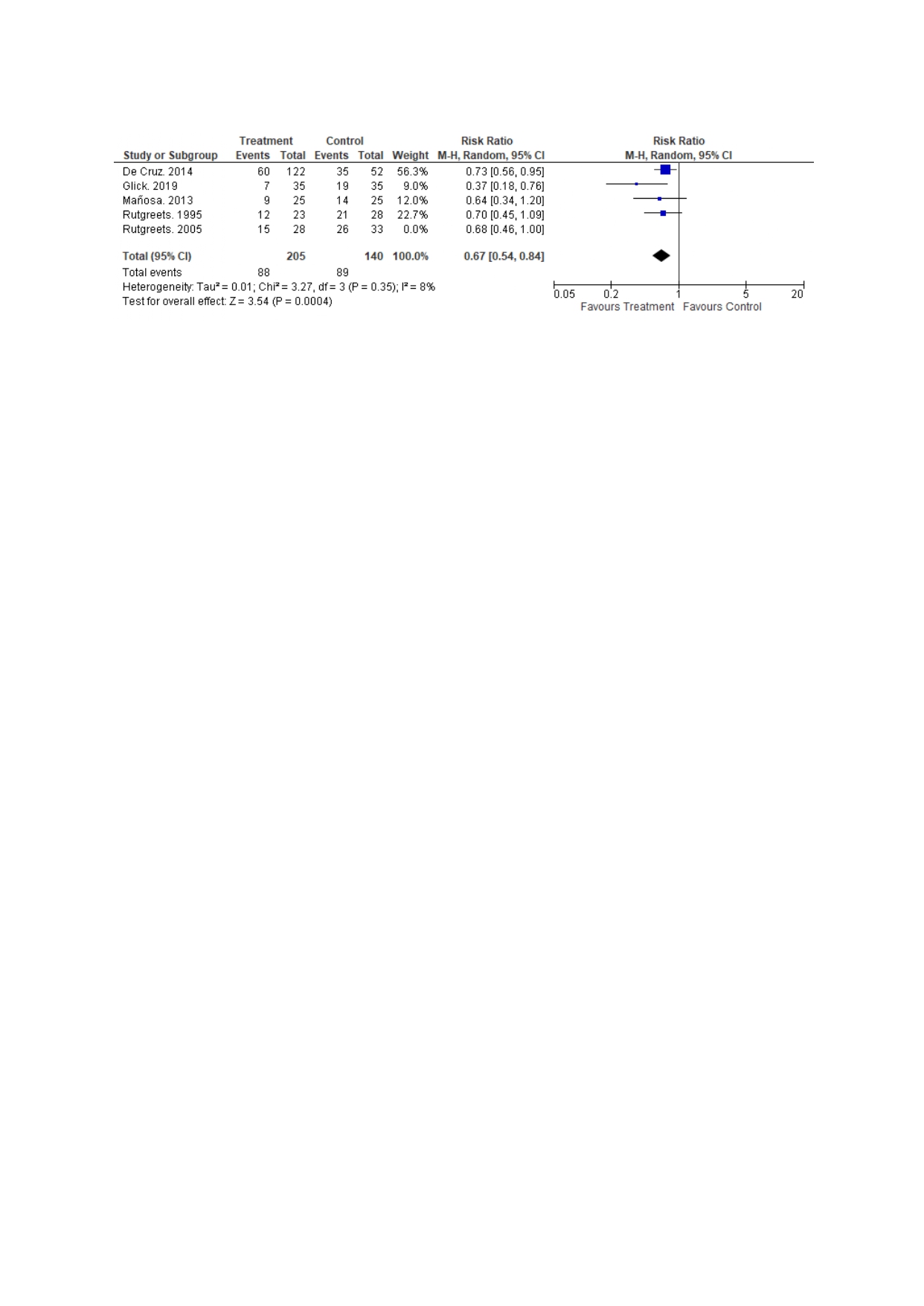
Figure: Figure 1: Comparison of endoscopic recurrence betwee metronidazole and placebo
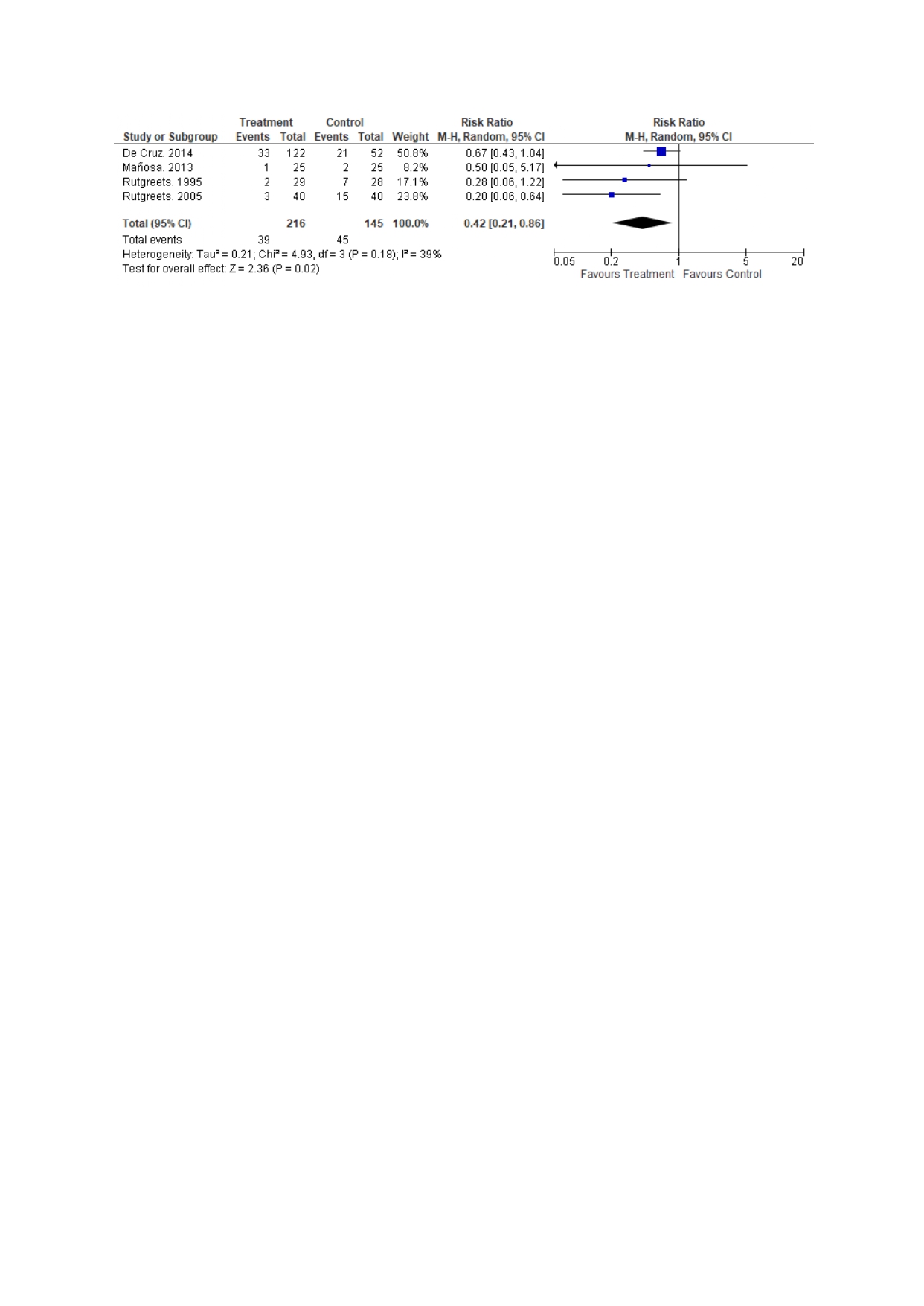
Figure: Figure 2: Comparison of clinical recurrence between the groups
Disclosures:
Aamir Saeed indicated no relevant financial relationships.
Saira Yousuf indicated no relevant financial relationships.
James Salughter indicated no relevant financial relationships.
Aimal Khan indicated no relevant financial relationships.
Alexander Hawkins indicated no relevant financial relationships.
David Schwartz: Abbvie – Consultant. Avobis – Consultant. Janssen – Consultant. Olympus – Consultant. Takeda – Consultant.
Sara Horst: Abbvie – Consultant. Biocon – Consultant. Celltrion – Consultant. J&J – Consultant. Pfizer – Consultant. Takeda – Consultant.
Baldeep Pabla: Astellas – Advisor or Review Panel Member. J&J – Advisory Committee/Board Member.
Aamir Saeed, MD1, Saira Yousuf, MD1, James Salughter, PhD1, Aimal Khan, MD1, Alexander Hawkins, MD1, David A. Schwartz, MD2, Sara Horst, MD, MPH, FACG3, Baldeep Pabla, MD, MSCI2. P1138 - Role of Nitroimidazoles in Crohn's Disease Recurrence in Patients Undergoing Ileocolonic Resection: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Vanderbilt University Medical Center, Nashville, TN; 2Vanderbilt Inflammatory Bowel Disease Clinic, Nashville, TN; 3Vanderbilt University School of Medicine, Nashville, TN
Introduction: Previous studies have shown mixed results regarding the efficacy of nitroimidazoles in preventing postoperative recurrence of CD. There is limited data to evaluate the efficacy of nitroimidazoles in post-operative recurrence rates in the era of increased biologic or advanced therapy use in the postoperative period for patients with inflammatory bowel disease. We aim to assess the endoscopic and clinical recurrence with the use of of nitroimidazole (metronidazole or ornidazole) in patients with CD undergoing ICR.
Methods: PubMed, Web of Science Core Collection, Embase, and the Cochrane Database of Systematic Reviews were reviewed to identify studies comparing outcomes of nitroimidazoles and placebo in CD recurrence in patients undergoing an ICR from inception until October 20, 2024. The primary outcome was endoscopic recurrence. The secondary outcomes included clinical recurrence and adverse events. We used the Mantel-Haenszel method to pool data for primary and secondary outcomes into random-effect model meta-analyses, we calculated pooled risk ratios (RRs), and their corresponding 95% confidence intervals (CIs) for all dichotomous variables, and the heterogeneity was assessed using the I2 statistic.
Results: Five studies (4 RCTS, 1 retrospective) comprising 432 patients (nitroimidazoles 250 and placebo 182) met inclusion criteria. The mean age was 35.2 years and 62% were male. Nitroimidazoles were associated with lower rates of endoscopic recurrence as compared to placebo at 12 months, 44% vs 66.5%, RR (95% CI): 0.68 (0.57, 0.81), p< 0.0001. Pooled rates for clinical recurrence were 18% and 31% between the groups, nitroimidazoles were associated with lower rates of clinical recurrence as compared to the placebo RR (95% CI): 0.42 (0.21, 0.86), p=0.02 (Figure 2). Nitroimidazoles were associated with lower adverse events as compared to the control group, with risk difference (95% CI): 0.20 (0.01, 0.39), p=0.04.
We also performed subgroup analysis among patients on metronidazole therapy vs placebo and 4 studies reported data on it. We found that metronidazole was associated with lower rates of endoscopic recurrence RR (95% CI): 0.67 (0.54, 0.84), p=0.0004 (Figure 1).
Discussion: Our findings observe that nitroimidazoles are associated with lower rates of endoscopic and clinical recurrence and adverse events as compared to placebo in CD recurrence in patients undergoing ICR.

Figure: Figure 1: Comparison of endoscopic recurrence betwee metronidazole and placebo

Figure: Figure 2: Comparison of clinical recurrence between the groups
Disclosures:
Aamir Saeed indicated no relevant financial relationships.
Saira Yousuf indicated no relevant financial relationships.
James Salughter indicated no relevant financial relationships.
Aimal Khan indicated no relevant financial relationships.
Alexander Hawkins indicated no relevant financial relationships.
David Schwartz: Abbvie – Consultant. Avobis – Consultant. Janssen – Consultant. Olympus – Consultant. Takeda – Consultant.
Sara Horst: Abbvie – Consultant. Biocon – Consultant. Celltrion – Consultant. J&J – Consultant. Pfizer – Consultant. Takeda – Consultant.
Baldeep Pabla: Astellas – Advisor or Review Panel Member. J&J – Advisory Committee/Board Member.
Aamir Saeed, MD1, Saira Yousuf, MD1, James Salughter, PhD1, Aimal Khan, MD1, Alexander Hawkins, MD1, David A. Schwartz, MD2, Sara Horst, MD, MPH, FACG3, Baldeep Pabla, MD, MSCI2. P1138 - Role of Nitroimidazoles in Crohn's Disease Recurrence in Patients Undergoing Ileocolonic Resection: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
