Sunday Poster Session
Category: IBD
P1124 - Fecal Microbiota vs Standard Medical Therapy in Inducing Histologic Remission in Ulcerative Colitis: A Systematic Review
Sunday, October 26, 2025
3:30 PM - 7:00 PM PDT
Location: Exhibit Hall
- HD
Harika Dadigiri, MD
NYMC at St Mary's and St Clare's
Pine Brook, NJ
Presenting Author(s)
Harika Dadigiri, MD1, Komal Arora, MBBS2, Hriday Shah, MD3, Canan Dirican, MD4, Pramil Cheriyath, MD5
1NYMC at St Mary's and St Clare's, Pine Brook, NJ; 2St. Mary's General Hospital, New York Medical College, Newark, NJ; 3NYMC Saint Clare's and Saint Mary's General Hospital, Harrison, NJ; 4NYMC Saint Clare's and Saint Mary's general Hospital, Pinebrook, NJ; 5NYMC Saint Clare's and Saint Mary's General Hospital, Denville, NJ
Introduction: Ulcerative colitis (UC) is a chronic inflammatory bowel disease that often requires long-
term immunosuppressive therapy or biologics for disease control. Fecal microbiota
transplantation (FMT), which aims to restore intestinal microbial balance, has emerged
as a promising therapeutic option in UC. However, evidence regarding its efficacy in
inducing histologic remission remains limited. This systematic review aims to evaluate
the efficacy of FMT in inducing clinical, endoscopic, and histologic remission in patients
with active UC.
Methods: Following PRISMA guidelines, a comprehensive literature search was conducted using
multiple databases, covering the last 10 years (2015–2025). We included randomized
controlled trials (RCTs) and open-label studies assessing clinical, endoscopic, and/or
histologic remission in adults with active UC receiving FMT versus standard therapy or
placebo. The primary outcome was clinical remission; secondary outcomes included
endoscopic and histologic remission. Quality assessment and data extraction were
performed independently by two reviewers.
Results: Five trials evaluated FMT for inducing remission in active UC. In the double-blind
LOTUS trial (2022; n = 35), corticosteroid-free clinical and endoscopic remission was
achieved in 53% of FMT recipients, compared to 15% in the placebo group (p = 0.027);
sustained endoscopic and histologic remission was observed at 56 weeks (100% in the
FMT group). In an open-label, factorial RCT (2022; n = 22), clinical remission at 6
weeks occurred in 67% of patients pretreated with antibiotics, compared to 18% of
those who were not (p = 0.065). A smaller RCT (2021; n = 12) reported clinical
remission in 33% of patients receiving encapsulated FMT, compared to placebo (p =
0.45). Two open-label capsule studies (2023; n = 21 and n = 22) reported clinical
remission rates of 57.1%, with endoscopic remission in 47.6%, and mycobiome shifts
(e.g., increased Kazachstania) associated with response.
Discussion: FMT appears to be an effective therapy for inducing clinical, endoscopic, and histologic
remission in patients with active ulcerative colitis. While these five trials revealed
promising results, larger and well-powered randomized trials are required to confirm
efficacy, optimize treatment protocols, and identify likely responders.
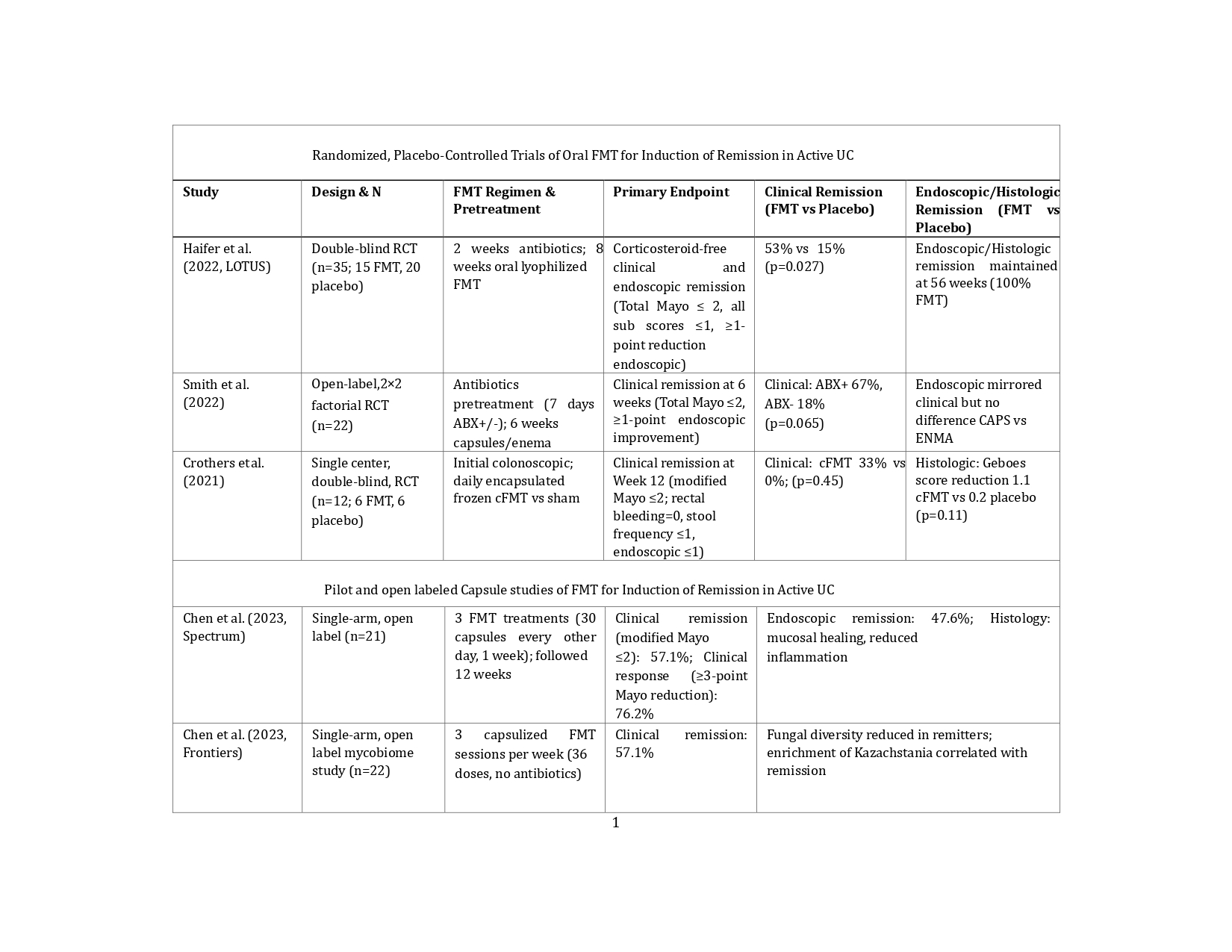
Figure: Table 1. Randomized, Placebo-Controlled Trials and Pilot Studies of Oral FMT for Induction of Remission in Active UC
Disclosures:
Harika Dadigiri indicated no relevant financial relationships.
Komal Arora indicated no relevant financial relationships.
Hriday Shah indicated no relevant financial relationships.
Canan Dirican indicated no relevant financial relationships.
Pramil Cheriyath indicated no relevant financial relationships.
Harika Dadigiri, MD1, Komal Arora, MBBS2, Hriday Shah, MD3, Canan Dirican, MD4, Pramil Cheriyath, MD5. P1124 - Fecal Microbiota vs Standard Medical Therapy in Inducing Histologic Remission in Ulcerative Colitis: A Systematic Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1NYMC at St Mary's and St Clare's, Pine Brook, NJ; 2St. Mary's General Hospital, New York Medical College, Newark, NJ; 3NYMC Saint Clare's and Saint Mary's General Hospital, Harrison, NJ; 4NYMC Saint Clare's and Saint Mary's general Hospital, Pinebrook, NJ; 5NYMC Saint Clare's and Saint Mary's General Hospital, Denville, NJ
Introduction: Ulcerative colitis (UC) is a chronic inflammatory bowel disease that often requires long-
term immunosuppressive therapy or biologics for disease control. Fecal microbiota
transplantation (FMT), which aims to restore intestinal microbial balance, has emerged
as a promising therapeutic option in UC. However, evidence regarding its efficacy in
inducing histologic remission remains limited. This systematic review aims to evaluate
the efficacy of FMT in inducing clinical, endoscopic, and histologic remission in patients
with active UC.
Methods: Following PRISMA guidelines, a comprehensive literature search was conducted using
multiple databases, covering the last 10 years (2015–2025). We included randomized
controlled trials (RCTs) and open-label studies assessing clinical, endoscopic, and/or
histologic remission in adults with active UC receiving FMT versus standard therapy or
placebo. The primary outcome was clinical remission; secondary outcomes included
endoscopic and histologic remission. Quality assessment and data extraction were
performed independently by two reviewers.
Results: Five trials evaluated FMT for inducing remission in active UC. In the double-blind
LOTUS trial (2022; n = 35), corticosteroid-free clinical and endoscopic remission was
achieved in 53% of FMT recipients, compared to 15% in the placebo group (p = 0.027);
sustained endoscopic and histologic remission was observed at 56 weeks (100% in the
FMT group). In an open-label, factorial RCT (2022; n = 22), clinical remission at 6
weeks occurred in 67% of patients pretreated with antibiotics, compared to 18% of
those who were not (p = 0.065). A smaller RCT (2021; n = 12) reported clinical
remission in 33% of patients receiving encapsulated FMT, compared to placebo (p =
0.45). Two open-label capsule studies (2023; n = 21 and n = 22) reported clinical
remission rates of 57.1%, with endoscopic remission in 47.6%, and mycobiome shifts
(e.g., increased Kazachstania) associated with response.
Discussion: FMT appears to be an effective therapy for inducing clinical, endoscopic, and histologic
remission in patients with active ulcerative colitis. While these five trials revealed
promising results, larger and well-powered randomized trials are required to confirm
efficacy, optimize treatment protocols, and identify likely responders.

Figure: Table 1. Randomized, Placebo-Controlled Trials and Pilot Studies of Oral FMT for Induction of Remission in Active UC
Disclosures:
Harika Dadigiri indicated no relevant financial relationships.
Komal Arora indicated no relevant financial relationships.
Hriday Shah indicated no relevant financial relationships.
Canan Dirican indicated no relevant financial relationships.
Pramil Cheriyath indicated no relevant financial relationships.
Harika Dadigiri, MD1, Komal Arora, MBBS2, Hriday Shah, MD3, Canan Dirican, MD4, Pramil Cheriyath, MD5. P1124 - Fecal Microbiota vs Standard Medical Therapy in Inducing Histologic Remission in Ulcerative Colitis: A Systematic Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
